Biophotonics: from nanoscience to the living world, and vice versa.
The Biodyn team investigates photochemical processes in organic molecules or nano-materials designed to probe biomolecular interactions, or to mimic – in material science – efficient photoactivated processes observed in natural biomolecular systems. Possible applications are in connection with molecular biology, health, or light-to-energy conversion.
Ultrafast Energy transfer in organic nano-antennas
We investigate the electronic energy transport mechanisms in dye-loaded, polymeric, organic nanoparticles (ONPs), which behave like synthetic light-harvesting antennas [1]. These ONPs are highly interesting for applications in biosensing, bio-imaging, or light-to-chemical energy conversion. Their photophysical properties are governed by exciton – i.e. excitation energy – diffusion and interactions within the ONP volume. We show in Figure 1, a representation of the chromophores encapsulated by the polymer and the energy diagrams for the involved exciton interactions.
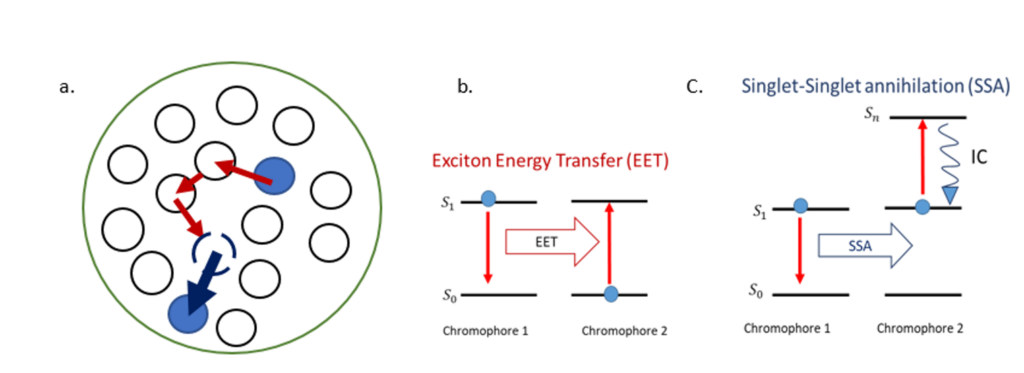
Following optical excitation, efficient exciton diffusion within the chromophores occurs via Exciton Energy Transfer (EET) as represented in the energy diagram in Figure 1.b. The exciton hops from one site to the nearest neighbors until it decays to the ground state, or until it interacts with another exciton, causing singlet-singlet annihilation (SSA). The latter process involves two excited chromophores: one decays to the ground state while the second is excited to an upper-lying singlet state and rapidly goes back to the first singlet excited state by internal conversion (IC) as shown in Figure 1.c. We aim to characterize the processes described above that are occurring on different time scales within the dye-loaded organic nanoparticles. To that end, we use different types of time-resolved fluorescence spectroscopies available in our lab. The EET between the nearest neighbours is estimated to occur on a femtosecond time scale. We retrieve it by using fluorescence up-conversion spectroscopy with polarization-resolved excitation and detection. Moreover, SSA reveals the time scale for exciton diffusion across the ONP, estimated to occur on a picosecond time scale. We monitor the SSA process using a confocal detection scheme and a streak camera.
Link to the ANR project LHNanoMat
Fluorescent probes
The photo reactivity of (bio)molecules is very generally influenced by the interaction with the local environment in the condensed phase (e.g. solvent, protein cavity, polymer matrix, etc.). This influence is exploited in so-called fluorescent probes, for which the fluorescence lifetime or spectrum may be very strongly affected by the interaction with the environment. One such example is the FRET process, in which the fluorescent «probe» or «donor» may undergo an ultrafast Förster Resonant Energy Transfer to a nearby energy «acceptor». Monitoring the change in the fluorescence lifetime of the donor reveals the proximity of the acceptor on a nanometer scale, and may be used to monitor structural changes of biomolecules labelled with a donor-acceptor FRET pair [2]. Alternatively, Excited State (intramolecular) Proton Transfer (ESPT) is another, possibly ultrafast, photoreaction that may quench the «probe» fluorescence to produce an excited-state tautomer with a very different fluorescence spectrum [3]. Such a fluorescent probe is precious to monitor changes in the local hydrogen-bond network in which it is involved, and may be used to detect – by fluorescence spectroscopy – changes in a protein folding state. (Ultrafast) Electron Transfer (ET) is another photoreaction [4] that may strongly modify the fluorescence properties of the electron donor depending on its relative orientation and distance from an electron acceptor (e.g. stacking interaction). Natural amino-acids like Tryptophan [5], or synthetic nucleotides like 2-anmino-purine [6] are UV fluorophores which have been widely used to report on local protein or DNA structure respectively, because their fluorescence lifetime is strongly affected by ET to nearby amino acids or nucleotides.
We have long been applying time-resolved fluorescence and absorption spectroscopy, in collaboration with biophysicists, biomolecular scientists, biochemists, or quantum chemists, in order to reveal the photoreaction dynamics of such environment-sensitive fluorophore, and (i) learn about the structure and interactions of fluorescently labeled biomolecules [2], (ii) contribute to the development of new fluorescent probes [3], (iii) explore the color-tuning mechanism of bioluminescent proteins [7] or (iv) investigate the use of light up-conversion in nanoparticles for photo-induced drug delivery [8] (LightlnDR ANR project).
References:
[1] A. Reisch, P. Didier, L. Richert, S. Oncul, Y. Arntz, Y. Mély, and A.S. Klymchenko, “Collective fluorescence switching of counterion-assembled dyes in polymer nanoparticles,” Nature Communications, vol. 5, no. 1,pp. 1–9, 2014.
[2] N. Grytsyk, D. Cianfarani, O. Crégut, L. Richert, C. Boudier, N. Humbert, P. Didier, Y. Mély, & J. Léonard. Kinetics of Protein-Assisted Nucleic Acid Interconversion Monitored by Transient Time-Resolved Fluorescence in Microfluidic Droplets ». Nucleic Acids Research 49, no 19 (8 novembre 2021): e111‑e111. https://doi.org/10.1093/nar/gkab687.
[3] A. Skilitsi, D. Agathangelou, I. Shulov, J. Conyard, S. Haacke, Y. Mély, A. Klymchenko, & J. Léonard. Ultrafast photophysics of the environment-sensitive 4[prime or minute]-methoxy-3-hydroxyflavone fluorescent dye . Physical Chemistry Chemical Physics 20, no 11 (2018): 7885‑95. https://doi.org/10.1039/C7CP08584B.
[4] L., Hoang‐Ngoan, J. Brazard, G. Barnoin, S. Vincent, B.Y. Michel, J. Léonard, & A. Burger. Control of Intermolecular Photoinduced Electron Transfer in Deoxyadenosine‐Based Fluorescent Probes. Chemistry – A European Journal 27, no 4 (18 janvier 2021): 1364‑73. https://doi.org/10.1002/chem.202003456.
[5] J. Léonard, D. Sharma, B. Szafarowicz, K. Torgasin, & S. Haacke. Formation Dynamics and Nature of Tryptophan’s Primary Photoproduct in Aqueous Solution . Physical Chemistry Chemical Physics 12, no 48 (2010): 15744. https://doi.org/10.1039/c0cp00615g.
[6] K. Voltz, J. Léonard, P. T. Touceda, J. Conyard, Z. Chaker, A. Dejaegere, J. Godet, Y. Mély, S. Haacke, & R. H. Stote. Quantitative Sampling of Conformational Heterogeneity of a DNA Hairpin Using Molecular Dynamics Simulations and Ultrafast Fluorescence Spectroscopy . Nucleic Acids Research 44, no 7 (20 avril 2016): 3408‑19. https://doi.org/10.1093/nar/gkw077.
[7] P. Gosset, G. Taupier, O. Crégut, J. Brazard, Y. Mély, K. Dorkenoo, J. Léonard, & P. Didier. Excited-State Proton Transfer in Oxyluciferin and Its Analogues . The Journal of Physical Chemistry Letters 11, no 9 (7 mai 2020): 3653‑59. https://doi.org/10.1021/acs.jpclett.0c00839.
[8] B. Anaïs, J. Chaud, J. Léonard, F. Bolze, S. Chassaing, B. Frisch, B. Heurtault, A. Kichler, & A Specht. Red Light-Responsive Upconverting Nanoparticles for Quantitative and Controlled Release of a Coumarin-Based Prodrug. Adv. Healthcare Mater. 2023, 12, 2201474. https://doi-org.scd-rproxy.u-strasbg.fr/10.1002/adhm.202201474
