Different surface chemistry methods are used to functionalize and seld-assemble our NPs. These methods include:
- Phase transfer method to ensure ligand exchange for instance in phophonate/citrate surface modification
- Grafting versatile end-groups by click-chemistry, silanization, phosphatation
- Protective cappings : wrapping, silica and polymers
1. Surface chemistry methods for iron oxide NPs
2. Surface chemistry methods for mesoporous silica NPs
Surface chemistry for MS is particularly appealing because of the possibility to graft siloxane bearing various end group which ensures versatile post-functionalization. In our team we have applied several methods comprising: a) siloxane grafting with different end-groups, b) ligand or NP post grafting and c) protective capping with coated silica and polymer layers.
A. Siloxane grafting (condensation to silica)
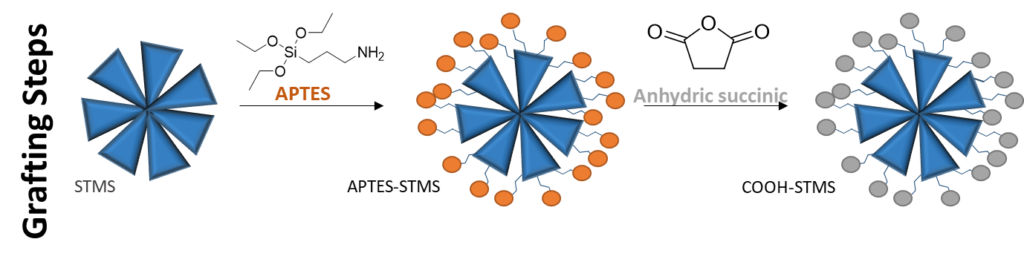
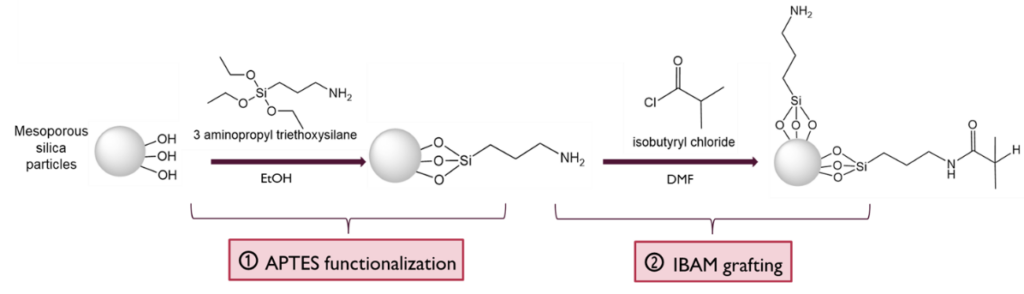
Here the MS surface allows grafting of siloxane bearing aminopropyltriethoxysilane (APTES), that can be converted into carboxylic acid COOH, or isobutyramide (IBAM). This grafting allows high surface density (1-3 molecules/nm2). Such surface chemistries allows thus different applications: loading drug or proteins by simple adsorption, or grafting ligand and imaging agents by covalent coupling.
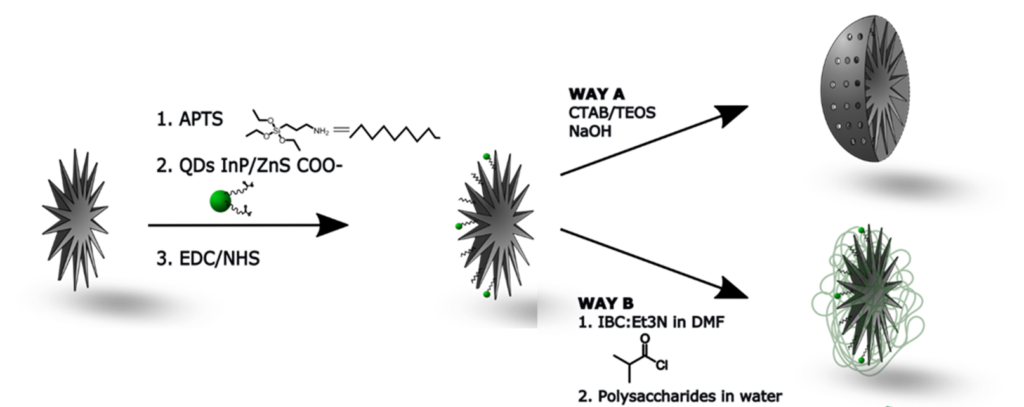
B. Ligand grafting (post-functionalization)
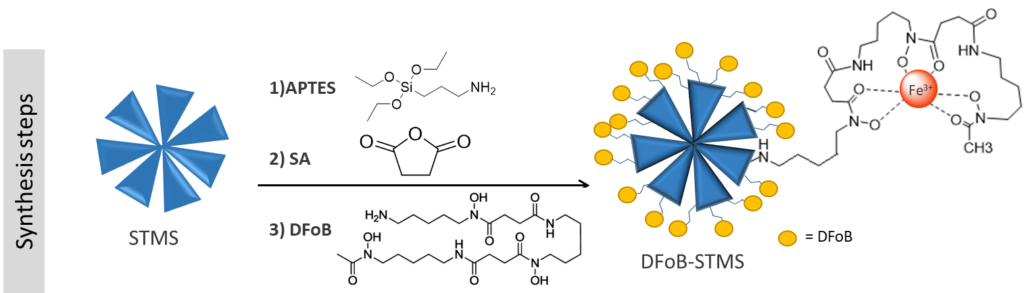
Additional functions can be covalently linked to the previous surface chemistry such as fluorophores or specific ligands. In one work, we have conjugated the iron chelatant desferrioxamine to the surface through carbo-diimide chemistry.
C. Protective capping with silica and polymers
After surface chemical modification, such MS NPs can be loaded with antitumor drugs or covalently linked with small fluorescent NPs such as Quantum dots for theranostic applications. (see part Nanomedecine applications).
With the aim to limit the leaking of antitumor drugs or heavy metal elements from QDs, strategies making use of silica or polymers coatings can be used. Biomacromolecules such as chitosan, dextran or HSA adsorbed on the IBAM-functionalized surface were shown to form a tight biopolymer shell around the NPs limiting drugs or heavy metal leaking.