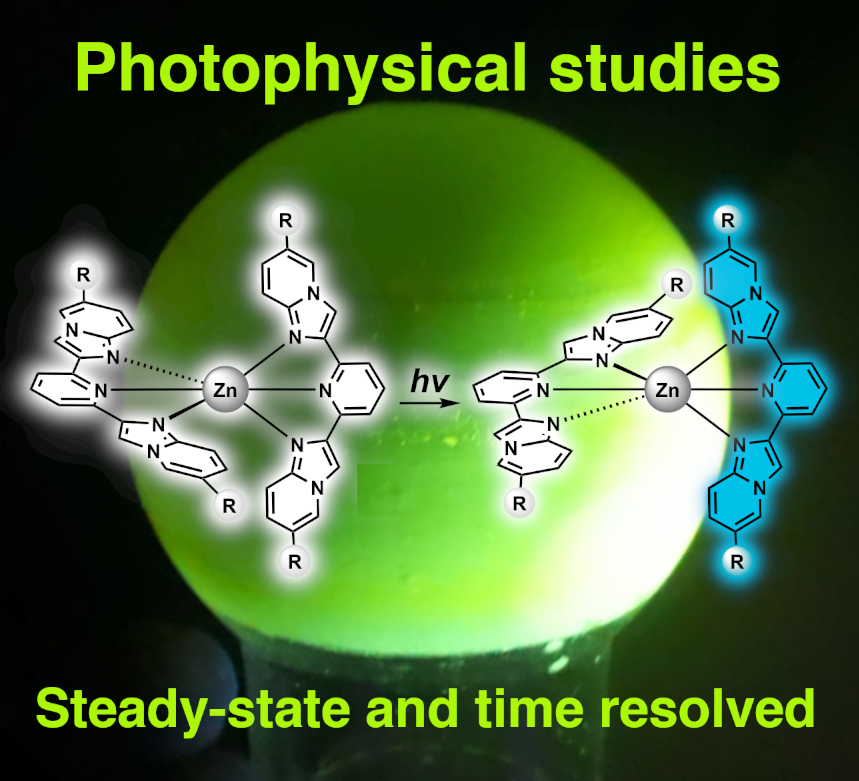
The activity of our équipe within the Department of Organic Materials (DMO) is mostly centered on the design and characterization of organometallic molecular and supramolecular systems for catalytic, photophysical/photoelectronic and materials applications. Our research, carried out in collaboration with numerous other research groups in France, Europe and worldwide, aims to deepen the knowledge on the correlation between structure and function, whether it is catalytic activity, selectivity, fluorescence colour or efficiency, redox behavour, etc.
< Back to Research Teams of the DMO
Research Activities
In the news We recently prepared and characterized a large family of chiral Re(I) N-heterocyclic carbene complexes that show circularly polarized luminescence and carried out an extensive theoretical study to better correlate their CPL emission to their structural, electronic and magnetic nature.10.1039/d3cp04300b
We recently studied a large series of (electro)luminescent binuclear biphenyl C^C Au(III) complexes which show anticancer activity and potential applications in the fabrication of light-emitting electrochemical cells.10.1021/acs.inorgchem.2c04293 10.1002/cplu.202300303
We recently presented the synthesis of three new tetranuclear copper-iodide clusters with cubane-like {Cu4 I4 } motif that show very intense yellow solid state luminescence (up to 83% PLQY). 10.3390/sym15061210
[ Read more ]
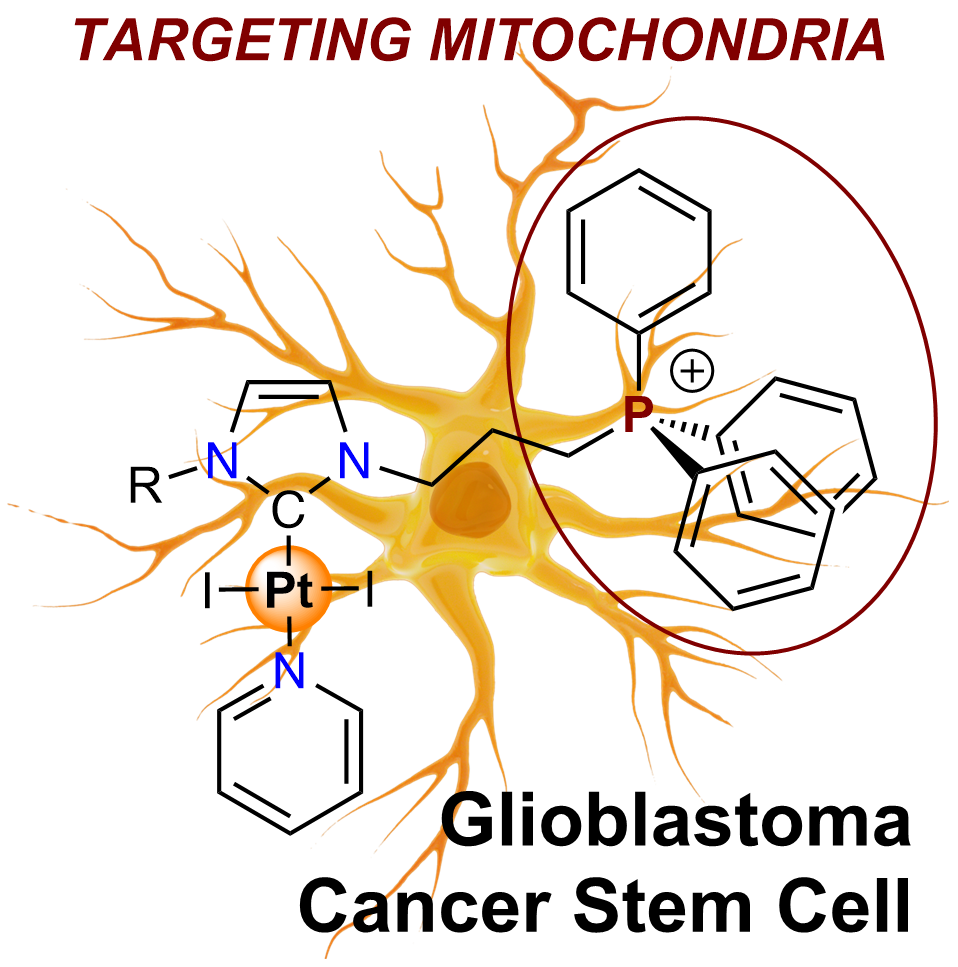
Bio-inorganic and medicinal chemistry Since the discovery of cisplatin as an anticancer agent, chemists have varied the ligands around the metal to improve its efficacy while trying to reduce its side effects. Although there have been more failures than successes, essential progress has been made in elucidating the mechanisms of tumor resistance properties. N-heterocyclic carbene as a ligand for organometallic chemistry is a relatively young field that offers new opportunities in many areas, including medicinal chemistry. In the laboratory, we are developing new tools to combat cancer cells and cancer stem cells. Our strategy combines noble metal with novel NHC ligands . In collaboration with the Faculty of Pharmacy (3BIO team ), we are studying their anticancer activity and mode of action.
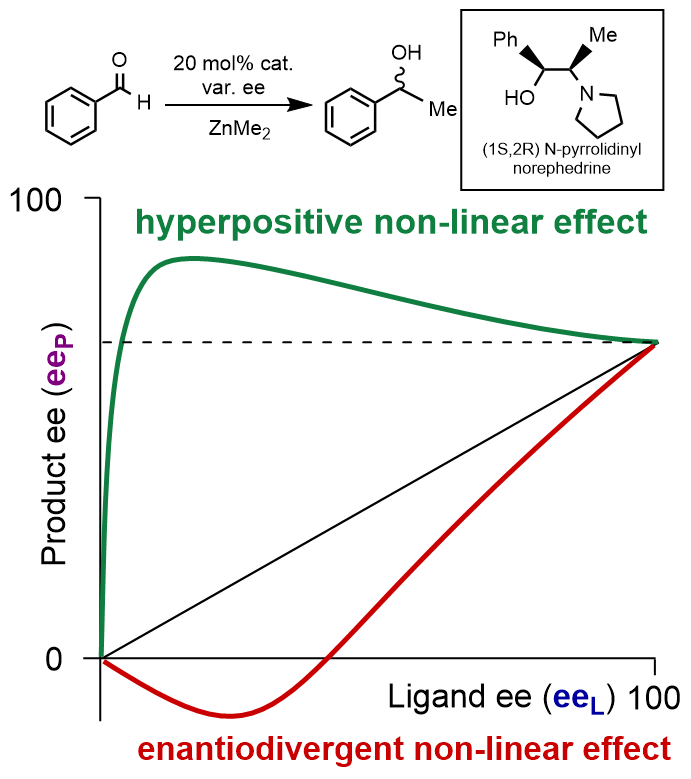
Organometallic chemistry and catalysis Asymmetric catalysis, which combines a transition metal with a chiral ligand, has emerged as the most elegant and efficient technique for creating a new stereogenic centre. As the catalyst is not consumed during the process, it can be used in substoichiometric quantities, improving efficiency and avoiding waste. In the laboratory, we design and synthesize new ligands for various enantioselective and non-enantioselective reactions . We are particularly interested in the phenomen of chirality amplification , which is essential not only to understand the mechanisms of the catalytic reaction, but also to contribute to the debate on homochirality in life.
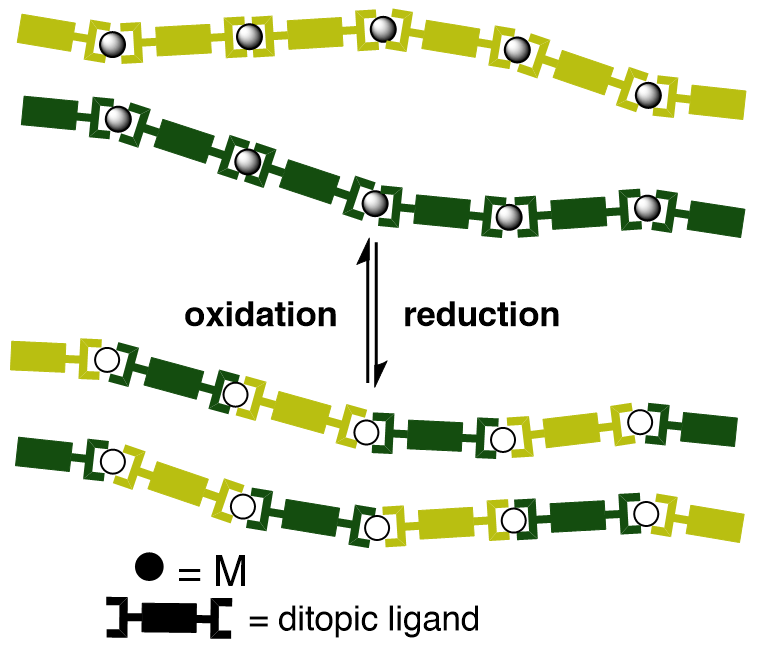
Supramolecular and materials chemistry Supramolecular functional materials capable of responding to external stimuli offer advantages over their conventional covalent counterparts. In our lab, the design of (chiral) molecular building blocks that self-assemble via intermolecular forces to form coordination structures enables us to create innovative materials for diverse applications. In particular, we are interested in the preparation of actuators capable of responding to external stimuli in a spatio-temporal manner, of self-repairing and of indicating a direction of movement. In addition to first-row transition metals such as Cu(I)/Cu(II) and Zn(I)/Zn(II), second and third-row metals with tendency to self-assemble and stack (mainly Pt(II) coordination compounds) are also prepared and studied, with a special focus on their luminescence properties.
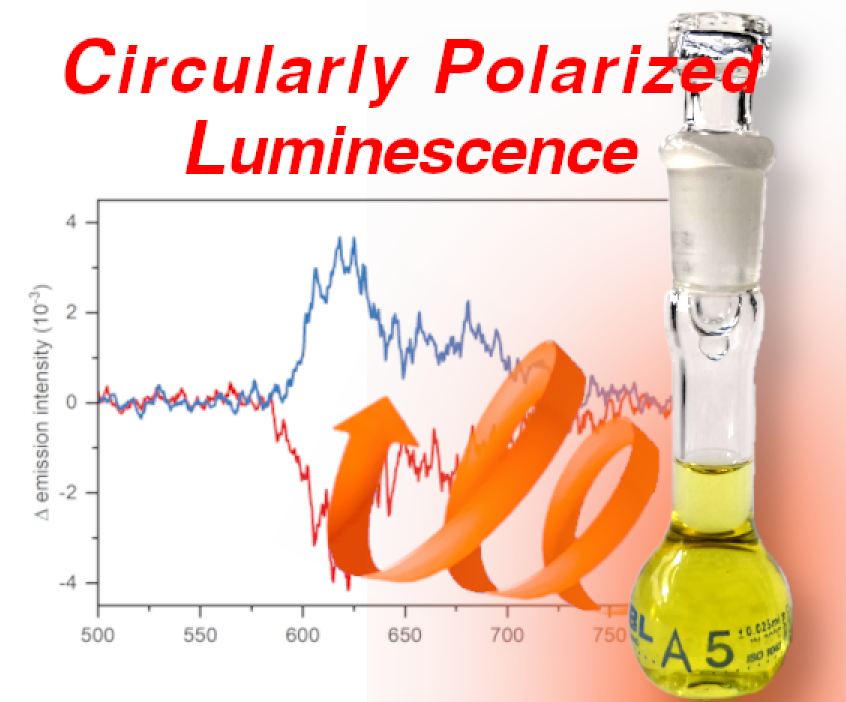
Red, Near-IR and circularly polarized emitters Our équipe is also working on the design, development and photophysical characterization of fluorescent and phosphorescent organometallic emitters pushing the boundaries of emission well above the red region of the visible spectrum, in the near-IR (>750 nm) . In addition to this, some of these compounds are designed with the goal to achieve circularly polarized luminescence (CPL) . These novel emitters are then fully characterized and sent to our collaborators to be tested in experimental devices.
Detailed photophysical studies In our laboratories we have access to a wide range of instruments to fully characterize luminescent compounds, with three double-beam UV-Vis spectrophotometers , a steady-state Horiba Jobin−Yvon IBH FL-322 Fluorolog 3 spectrometer equipped with a 450 W xenon arc lamp, and time-resolved PicoQuant FluoTime 300 fluorimeter with multiple pulsed lasers. With the aid of custom quartzware we can carry out extensive photophysical studies in solution, at low temperature, in the solid state, in spin-coated polymer thin films, and in degassed conditions. This allows us to engage in multiple collaborations with researchers who wish to study and investigate the luminescence properties and emission dynamics of their organic, organometallic or inorganic compounds.
Experimental Capabilities
Catalysis Our laboratories are equipped for catalyst preparation and characterization, as well as for catalysis control. We also have routine techniques for conducting analyses (GC-MS, chiral GC, chiral HPLC, etc.).
Organometallics The laboratory has state-of-the-art equipment for the synthesis of transition metal chemistry including Schlenk vacuum lines for handling extremely air-sensitive compounds, dry-box and Solvent Purification System.
Photophysic sWe are equipped to carry out in-depth photophysical studies with the aid of commercial and custom quartzware, a turbomolecular pump, and all the instruments in the Optical Characterization platform of the IPCMS.
Team Members
Stéphane BELLEMIN-LAPONNAZ
Prior Members
Patents
NEW TITANIUM CATALYST FOR THE POLYESTER MANUFACTURING PROCESSEP4101817A1 • 2022-12-14 • CLARIANT INT LTD [CH]
PHOSPHONITE COMPOUNDS AS PROCESS STABILIZERSUS11767413B2 (A1) • 2023-09-26 • CLARIANT INT LTD [CH]
TETRA-NUCLEAR NEUTRAL COPPER (I) COMPLEXESEP3810619A1 (B1) • 2021-04-28 • CLARIANT INT LTD [CH]
TETRA-NUCLEAR NEUTRAL COPPER (I) COMPLEXES WITH DIARYLPHOSPHINE LIGANDSWO2019243581A1 • 2019-12-26 • CLARIANT PLASTICS & COATINGS LTD [CH]
Recent publications :
1839302
ITCCYZMF
2024
1
surface-science-reports
50
creator
asc
year
10207
https://www.ipcms.fr/plugins/zotpress/
%7B%22status%22%3A%22success%22%2C%22updateneeded%22%3Afalse%2C%22instance%22%3Afalse%2C%22meta%22%3A%7B%22request_last%22%3A0%2C%22request_next%22%3A0%2C%22used_cache%22%3Atrue%7D%2C%22data%22%3A%5B%7B%22key%22%3A%227RMU8JMF%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Appleton%20et%20al.%22%2C%22parsedDate%22%3A%222024%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EJ.L.%20Appleton%2C%20L.%20Ballerini%2C%20S.%20Choua%2C%20C.%20Gourlaouen%2C%20R.%20Ruppert%2C%20M.%20Mauro%2C%20Cooperative%2C%20Close%20and%20Remote%20Steric%20Effects%20on%20the%20Structural%20and%20Optical%20Properties%20of%20Copper%28I%29%20Bis-Phenanthroline%20Complexes%2C%20European%20Journal%20of%20Inorganic%20Chemistry%2027%20%282024%29%20e202400278.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fejic.202400278%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fejic.202400278%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Cooperative%2C%20Close%20and%20Remote%20Steric%20Effects%20on%20the%20Structural%20and%20Optical%20Properties%20of%20Copper%28I%29%20Bis-Phenanthroline%20Complexes%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jordan%20L.%22%2C%22lastName%22%3A%22Appleton%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Lavinia%22%2C%22lastName%22%3A%22Ballerini%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sylvie%22%2C%22lastName%22%3A%22Choua%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Christophe%22%2C%22lastName%22%3A%22Gourlaouen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Romain%22%2C%22lastName%22%3A%22Ruppert%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matteo%22%2C%22lastName%22%3A%22Mauro%22%7D%5D%2C%22abstractNote%22%3A%22The%20synthesis%20as%20well%20as%20the%20structural%2C%20optical%20and%20computational%20characterization%20of%20seven%20new%20highly%20hindered%20homoleptic%20copper%28I%29%20phenanthroline%20complexes%20%28namely%20C1-C7%29%20is%20reported%20along%20with%20the%20comparison%20with%20the%20benchmark%20derivatives.%20Despite%20limited%20steric%20hindrance%20in%20direct%20proximity%20of%20the%20copper%28I%29%20coordination%20site%2C%20X-ray%20structures%20show%20that%20the%20higher%20hindered%20derivatives%20of%20the%20series%20display%20a%20more%20optimal%20tetrahedral%20geometry%20with%20minimal%20D2%20symmetry%20distortion.%20A%20novel%20remote%20control%20of%20the%20geometry%2C%20with%20steric%20hindrance%20away%20from%20the%20coordination%20site%2C%20leads%20to%20a%20favorable%20arrangement%20as%20demonstrated%20by%20structural%20and%20computational%20data.%20In%20addition%2C%20electrochemical%20and%20steady-state%20and%20time-resolved%20photophysical%20studies%20are%20presented%20which%20further%20support%20the%20beneficial%20effects%20on%20the%20ground-state%20redox%20and%20excited-state%20properties%20when%20both%20remote%20and%20close%20steric%20effects%20are%20exploited.%20Indeed%2C%20both%20an%20increase%20of%20the%20photoluminescence%20quantum%20yield%20and%20a%20prolongation%20of%20the%20excited%20state%20lifetime%20is%20observed%20for%20the%20highly-substituted%20derivative%20C6%20compared%20to%20benchmark%20counterparts%20on%20account%20of%20the%20reduced%20excited-state%20flattening%20distortions%20imparted%20by%20the%20additional%20steric%20constraints.Seven%20copper%28I%29%20phenanthroline%20complexes%20have%20been%20synthesized%20with%20steric%20hindrance%20present%20at%20and%20away%20from%20the%20coordination%20site%2C%20coined%20as%20remote%20control.%20This%20technique%20allowed%20for%20the%20synthesis%20of%20highly%20hindered%20stable%20homoleptic%20complexes%20which%20presented%20interesting%20ground%20and%20excited%20state%20properties%20that%20were%20corroborated%20by%20DFT%20calculations.%20image%22%2C%22date%22%3A%222024%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1002%5C%2Fejic.202400278%22%2C%22ISSN%22%3A%221434-1948%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1002%5C%2Fejic.202400278%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222024-09-13T09%3A14%3A15Z%22%7D%7D%2C%7B%22key%22%3A%22M3XBAN5I%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Ballerini%20et%20al.%22%2C%22parsedDate%22%3A%222024%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EL.%20Ballerini%2C%20W.-M.%20Zhang%2C%20T.%20Groizard%2C%20C.%20Gourlaouen%2C%20F.%20Polo%2C%20A.%20Jouaiti%2C%20H.-C.%20Su%2C%20M.%20Mauro%2C%20Binuclear%20iridium%28iii%29%20complexes%20for%20efficient%20near-infrared%20light-emitting%20electrochemical%20cells%20with%20electroluminescence%20up%20to%20800%20nm%2C%20Journal%20of%20Materials%20Chemistry%20C%2012%20%282024%29%2012769%26%23x2013%3B12783.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fd4tc02040e%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fd4tc02040e%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Binuclear%20iridium%28iii%29%20complexes%20for%20efficient%20near-infrared%20light-emitting%20electrochemical%20cells%20with%20electroluminescence%20up%20to%20800%20nm%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Lavinia%22%2C%22lastName%22%3A%22Ballerini%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Wei-Min%22%2C%22lastName%22%3A%22Zhang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thomaz%22%2C%22lastName%22%3A%22Groizard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Christophe%22%2C%22lastName%22%3A%22Gourlaouen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Federico%22%2C%22lastName%22%3A%22Polo%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Abdelaziz%22%2C%22lastName%22%3A%22Jouaiti%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Hai-Ching%22%2C%22lastName%22%3A%22Su%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matteo%22%2C%22lastName%22%3A%22Mauro%22%7D%5D%2C%22abstractNote%22%3A%22Near%20infrared%20%28NIR%29%20emitting%20optoelectronic%20devices%20have%20great%20potential%20for%20applications%20in%20communication%2C%20encryption%20technologies%2C%20night-vision%20display%20and%20photodynamic%20biomedical%20devices.%20Nevertheless%2C%20their%20development%20is%20currently%20hampered%20by%20the%20lack%20of%20efficient%20NIR-emissive%20materials.%20Herein%2C%20a%20novel%20class%20of%20cationic%20binuclear%20Ir%28iii%29%20emitters%20%28Ir-D1%20and%20Ir-D2%29%20based%20on%20a%20ditopic%20coordinating%20scaffold%20featuring%20the%20pi-deficient%20thiazolo%5B5%2C4-d%5Dthiazole%20and%20pi-accepting%20moiety%20%28either%20pyridine%20or%20pyrazine%29%2C%20is%20described%20and%20fully%20characterized%20using%20photophysical%20and%20computational%20techniques.%20Comparison%20with%20the%20parental%20mononuclear%20derivatives%20Ir-M1%20and%20Ir-M2%20is%20provided%20as%20well.%20Remarkably%2C%20the%20binuclear%20complexes%20display%20NIR%20photoluminescence%20in%20solution%20with%20a%20maximum%20up%20to%20lambda%28em%29%20%3D%20ca.%20840%20nm%2C%20which%20represent%20some%20rare%20examples%20of%20metal%20complexes%20emitting%20in%20this%20spectral%20region.%20Interestingly%2C%20NIR%20photoluminescence%20is%20retained%20in%20polymer-matrix%20thin-film%20for%20the%20binuclear%20counterparts.%20These%20findings%20prompt%20the%20successful%20use%20of%20these%20NIR%20emitters%20as%20electroactive%20materials%20in%20light%20emitting%20electrochemical%20cells%20%28LECs%29.%20Binuclear%20complexes%20Ir-D1%20and%20Ir-D2%20yield%20electroluminescence%20peaking%20at%20lambda%28EL%29%20%3D%20750%20and%20800%20nm%2C%20respectively%2C%20and%20device%20performances%20that%20are%20the%20highest%20reported%20for%20LECs%20in%20this%20spectral%20region%20to%20date%20for%20molecular%20%28i.e.%20non-excimer%29%20emitters.%20This%20work%20demonstrates%20the%20superior%20performances%20of%20the%20binuclear%20design%20strategy%20for%20achieving%20efficient%20NIR%20electroluminescence.%22%2C%22date%22%3A%222024%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1039%5C%2Fd4tc02040e%22%2C%22ISSN%22%3A%222050-7526%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1039%5C%2Fd4tc02040e%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222025-02-14T13%3A40%3A54Z%22%7D%7D%2C%7B%22key%22%3A%22T7DG4TFG%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Bellemin-Laponnaz%20and%20Achard%22%2C%22parsedDate%22%3A%222024%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ES.%20Bellemin-Laponnaz%2C%20T.%20Achard%2C%20Recent%20Progress%20in%20Developing%20Thioether-Containing%20Ligands%20for%20Catalysis%20Applications%2C%20Synthesis-Stuttgart%2056%20%282024%29%201369%26%23x2013%3B1380.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1055%5C%2Fa-2193-4927%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1055%5C%2Fa-2193-4927%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Recent%20Progress%20in%20Developing%20Thioether-Containing%20Ligands%20for%20Catalysis%20Applications%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thierry%22%2C%22lastName%22%3A%22Achard%22%7D%5D%2C%22abstractNote%22%3A%22The%20ligand%20that%20stabilizes%20the%20metal%20center%20is%20crucial%20to%20its%20catalytic%20activity.%20Historically%20dominated%20by%20phosphorus%20and%20nitrogen%2C%20sulfur%20has%20long%20been%20little%20considered%20as%20a%20hetero%20element%20for%20stabilizing%20a%20potentially%20active%20metal%20center.%20However%2C%20this%20situation%20is%20changing%20and%20we%20are%20seeing%20more%20and%20more%20examples%20that%20incorporate%20this%20element.%20This%20review%20provides%20an%20overview%20of%20recent%20transition-metal-catalyzed%20reactions%20with%20ligands%20containing%20neutral%20sulfur%20groups%2C%20i.e.%20thioethers.%20A%20selection%20of%20examples%20published%20since%202013%20illustrates%20the%20diversity%20of%20applications%20of%20thioether-containing%20ligands%20and%20shows%20that%20sulfur%20should%20be%20more%20widely%20used%20in%20the%20development%20of%20homogeneous%20catalysis.%22%2C%22date%22%3A%222024%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1055%5C%2Fa-2193-4927%22%2C%22ISSN%22%3A%220039-7881%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1055%5C%2Fa-2193-4927%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222025-02-14T13%3A01%3A45Z%22%7D%7D%2C%7B%22key%22%3A%2224NNYNZV%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Dussart%20et%20al.%22%2C%22parsedDate%22%3A%222024%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EC.%20Dussart%2C%20A.%20Maisse-Francois%2C%20S.%20Bellemin-Laponnaz%2C%20Chiral%20Self-Sorting%20Process%20With%20C2%20Symmetric%20Bisimidazoline%20Ligands%2C%20Chirality%2036%20%282024%29%20e23720.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fchir.23720%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fchir.23720%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Chiral%20Self-Sorting%20Process%20With%20C2%20Symmetric%20Bisimidazoline%20Ligands%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Caitlyn%22%2C%22lastName%22%3A%22Dussart%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Aline%22%2C%22lastName%22%3A%22Maisse-Francois%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%5D%2C%22abstractNote%22%3A%22We%20have%20studied%20the%20coordination%20chemistry%20of%20chiral%20imidazoline-based%20C-2-symmetric%20ligands%20with%20zinc%20%28II%29%20and%20copper%20%28II%29.%20Two%20types%20of%20bisimidazoline%20ligands%20were%20studied%2C%20one%20with%20the%20free%20amine%20%28BIM-H%29%20and%20the%20other%20with%20the%20amine%20protected%20by%20a%20toluene%20sulfonyl%20group%20in%20position%206%20%28BIM-Ts%29.%20The%20complexes%20formed%20were%20isolated%2C%20purified%2C%20and%20characterized%2C%20in%20particular%20by%20X-ray%20diffraction%20studies%20and%20CD%20in%20the%20case%20of%20the%20enantiopure%20complexes.%20By%20playing%20with%20the%20choice%20of%20ligand%20system%20%28enantiopure%20or%20racemate%29%2C%20we%20were%20able%20to%20show%20that%20the%20selective%20formation%20of%20homoleptic%20and%20heteroleptic%20metal%20complexes%20can%20be%20controlled%20by%20means%20of%20the%20chiral%20molecular%20instruction%20of%20bisimidazoline%20ligands.%22%2C%22date%22%3A%222024%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1002%5C%2Fchir.23720%22%2C%22ISSN%22%3A%220899-0042%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1002%5C%2Fchir.23720%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222024-11-08T10%3A37%3A11Z%22%7D%7D%2C%7B%22key%22%3A%22JNNK79VJ%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Dussart%20et%20al.%22%2C%22parsedDate%22%3A%222024%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EC.%20Dussart%2C%20A.%20Bonnefont%2C%20S.%20Bellemin-Laponnaz%2C%20Cu%26lt%3BSUP%26gt%3BI%26lt%3B%5C%2FSUP%26gt%3B%5C%2FCu%26lt%3BSUP%26gt%3BII%26lt%3B%5C%2FSUP%26gt%3B%20Chiral%20Homoleptic%20Complexes%3A%20Study%20of%20Self-Recognition%20and%20Self-Discrimination%2C%20European%20Journal%20of%20Inorganic%20Chemistry%2027%20%282024%29%20e202400527.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fejic.202400527%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fejic.202400527%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Cu%3CSUP%3EI%3C%5C%2FSUP%3E%5C%2FCu%3CSUP%3EII%3C%5C%2FSUP%3E%20Chiral%20Homoleptic%20Complexes%3A%20Study%20of%20Self-Recognition%20and%20Self-Discrimination%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Caitlyn%22%2C%22lastName%22%3A%22Dussart%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Bonnefont%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%5D%2C%22abstractNote%22%3A%22The%20formation%20of%20ML2%20homoleptic%20copper%28I%29%20and%20copper%28II%29%20complexes%20from%20bidentate%20chiral%20ligands%20with%20C2%20symmetry%2C%20comprising%20three%20types%20of%20substituents%20%28namely%20benzyl%2C%20indanyl%20and%20phenyl%20groups%29%2C%20was%20investigated.%20The%20formation%20of%20homochiral%20or%20heterochiral%20complexes%20can%20be%20influenced%20by%20the%20nature%20of%20the%20substituents%20and%20the%20geometry%20induced%20by%20the%20oxidation%20state%20of%20the%20metal.%20All%20the%20complexes%20were%20isolated%20and%20characterized%2C%20in%20particular%20in%20the%20solid%20state%20by%20X-ray%20diffraction%20studies.%20In%20solution%2C%20cyclic%20voltammetry%20was%20used%20to%20study%20ligand%20association%20and%20discrimination%20induced%20by%20the%20CuI%5C%2FCuII%20transition.%22%2C%22date%22%3A%222024%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1002%5C%2Fejic.202400527%22%2C%22ISSN%22%3A%221434-1948%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1002%5C%2Fejic.202400527%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222025-01-13T14%3A08%3A05Z%22%7D%7D%2C%7B%22key%22%3A%229XJHEV4I%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Giuso%20et%20al.%22%2C%22parsedDate%22%3A%222024%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EV.%20Giuso%2C%20C.%20Gourlaouen%2C%20M.%20Delporte-Pebay%2C%20T.%20Groizard%2C%20N.%20Vanthuyne%2C%20J.%20Crassous%2C%20C.%20Daniel%2C%20M.%20Mauro%2C%20Chiroptical%20activity%20of%20benzannulated%20N-heterocyclic%20carbene%20rhenium%28i%29%20tricarbonyl%20halide%20complexes%3A%20towards%20efficient%20circularly%20polarized%20luminescence%20emitters%2C%20Physical%20Chemistry%20Chemical%20Physics%2026%20%282024%29%204855%26%23x2013%3B4869.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fd3cp04300b%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fd3cp04300b%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Chiroptical%20activity%20of%20benzannulated%20N-heterocyclic%20carbene%20rhenium%28i%29%20tricarbonyl%20halide%20complexes%3A%20towards%20efficient%20circularly%20polarized%20luminescence%20emitters%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Valerio%22%2C%22lastName%22%3A%22Giuso%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Christophe%22%2C%22lastName%22%3A%22Gourlaouen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Mathias%22%2C%22lastName%22%3A%22Delporte-Pebay%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thomas%22%2C%22lastName%22%3A%22Groizard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nicolas%22%2C%22lastName%22%3A%22Vanthuyne%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jeanne%22%2C%22lastName%22%3A%22Crassous%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Chantal%22%2C%22lastName%22%3A%22Daniel%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matteo%22%2C%22lastName%22%3A%22Mauro%22%7D%5D%2C%22abstractNote%22%3A%22The%20design%20of%20enantiomerically%20pure%20circularly%20polarized%20luminescent%20%28CPL%29%20emitters%20would%20enormously%20benefit%20from%20the%20accurate%20and%20in-depth%20interpretation%20of%20the%20chiroptical%20properties%20by%20means%20of%20jointly%20%28chiroptical%29%20photophysical%20measurements%20and%20state-of-the-art%20theoretical%20investigation.%20Herein%2C%20computed%20and%20experimental%20%28chiro-%29optical%20properties%20of%20a%20series%20of%20eight%20enantiopure%20phosphorescent%20rhenium%28i%29%20tricarbonyl%20complexes%20are%20systematically%20compared%20in%20terms%20of%20electronic%20circular%20dichroism%20%28ECD%29%20and%20CPL.%20The%20compounds%20have%20general%20formula%20fac-%5BReX%28CO%29%283%29%28N%3C%5E%3EC-NHC%29%5D%2C%20where%20N%3C%5E%3EC-NHC%20is%20a%20pyridyl%20benzannulated%20N-heterocyclic%20carbene%20deriving%20from%20a%20%28substituted%29%202-%28pyridin-2-yl%29imidazo%5B1%2C5-a%5Dpyridin-2-ium%20proligand%20and%20X%20%3D%20Cl%2C%20Br%20and%20I%2C%20and%20display%20structured%20red%20phosphorescence%20with%20long-lived%20%28tau%20%3D%207.0-19.1%20mu%20s%29%20excited-state%20lifetime%20and%20dissymmetry%20factors%20%7Cg%28Lum%29%7C%20up%20to%204%20x%2010%28-3%29.%20The%20mixing%20of%20the%20character%20of%20the%20lowest-lying%20emitting%20triplet%20excited%20state%20is%20finely%20modulated%20between%20ligand%20centred%20%28%28LC%29-L-3%29%2C%20metal-to-ligand%20charge%20transfer%20%28%28MLCT%29-M-3%29%20and%20halogen-to-ligand%20charge%20transfer%20%28%28XLCT%29-X-3%29%20by%20the%20nature%20of%20the%20ancillary%20halogen%20and%20the%20chromophoric%20N%3C%5E%3EC-NHC%20ligand.%20The%20study%20unravels%20the%20effect%20exerted%20by%20the%20nature%20of%20the%20excited%20state%20onto%20the%20ECD%20and%20CPL%20activity%20and%20will%20help%20to%20pave%20the%20way%20to%20construct%20efficient%20CPL%20emitters%20by%20chemical%20design.%22%2C%22date%22%3A%222024%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1039%5C%2Fd3cp04300b%22%2C%22ISSN%22%3A%221463-9076%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1039%5C%2Fd3cp04300b%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222025-02-14T13%3A15%3A46Z%22%7D%7D%2C%7B%22key%22%3A%22G3EUMQBF%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Mechrouk%20et%20al.%22%2C%22parsedDate%22%3A%222024%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EV.%20Mechrouk%2C%20B.%20Leforestier%2C%20W.%20Chen%2C%20A.I.%20Poblador-Bahamonde%2C%20A.%20Maisse-Francois%2C%20S.%20Bellemin-Laponnaz%2C%20T.%20Achard%2C%20Diastereoselective%20Synthesis%20of%20Sulfoxide-Functionalized%20N-Heterocyclic%20Carbene%20Ruthenium%20Complexes%3A%20An%20Experimental%20and%20Computational%20Study%2C%20Chemistry-a%20European%20Journal%2030%20%282024%29%20e202401390.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fchem.202401390%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fchem.202401390%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Diastereoselective%20Synthesis%20of%20Sulfoxide-Functionalized%20N-Heterocyclic%20Carbene%20Ruthenium%20Complexes%3A%20An%20Experimental%20and%20Computational%20Study%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Victoria%22%2C%22lastName%22%3A%22Mechrouk%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Baptiste%22%2C%22lastName%22%3A%22Leforestier%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Weighang%22%2C%22lastName%22%3A%22Chen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Amalia%20I.%22%2C%22lastName%22%3A%22Poblador-Bahamonde%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Aline%22%2C%22lastName%22%3A%22Maisse-Francois%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thierry%22%2C%22lastName%22%3A%22Achard%22%7D%5D%2C%22abstractNote%22%3A%22The%20synthesis%20of%20sulfoxide-functionalized%20NHC%20ligand%20precursors%20were%20carried%20out%20by%20direct%20and%20mild%20oxidation%20from%20corresponding%20thioether%20precursors%20with%20high%20selectivity.%20Using%20these%20salts%2C%20a%20series%20of%20cationic%20%5BRu%28II%29%28eta%206-p-cymene%29%28NHC-SO%29Cl%5D%2B%20complexes%20were%20obtained%20in%20excellent%20yields%20by%20the%20classical%20Ag2O%20transmetallation%20route.%20NMR%20analyses%20suggested%20a%20chelate%20structure%20for%20the%20metal%20complexes%2C%20and%20X-ray%20diffractometry%20studies%20of%20complexes%204%20b%2C%204%20c%2C%204dBArF%20and%204%20e%20unambiguously%20confirmed%20the%20preference%20for%20the%20bidentate%20%28kappa%202-C%2CS%29%20coordination%20mode%20of%20the%20NHC-SO%20ligands.%20Interestingly%2C%20only%20one%20diastereomer%2C%20in%20the%20form%20of%20an%20enantiomeric%20pair%2C%20was%20observed%20both%20in%201H%20NMR%20and%20in%20the%20solid%20state%20for%20the%20complexes.%20DFT%20calculations%20showed%20a%20possible%20intrinsic%20energy%20difference%20between%20the%20two%20pairs%20of%20diastereomer.%20The%20calculated%20energy%20barriers%20suggested%20that%20inversion%20of%20the%20sulfoxide%20is%20only%20plausible%20from%20the%20higher%20energy%20diastereomer%20together%20with%20bulky%20substituents.%20Inverting%20the%20configuration%20at%20the%20Ru%20center%20instead%20shows%20a%20lower%20and%20accessible%20activation%20barrier%20to%20provide%20the%20most%20stable%20diastereomer%20through%20thermodynamic%20control%2C%20consistent%20with%20the%20observation%20of%20a%20single%20species%20by%201H%20NMR%20as%20a%20pair%20of%20enantiomers.%20All%20these%20complexes%20catalyse%20the%20beta-alkylation%20of%20secondary%20alcohols.%20Complex%204dPF6%20bearing%20an%20NHC-functionalised%20S-Ad%20group%20has%20been%20further%20studied%20with%20different%20primary%20and%20secondary%20alcohols%20as%20substrates%2C%20showing%20high%20reactivity%20and%20high%20to%20moderate%20beta-ol-selectivities.Diastereoselective%20synthesis%3A%20A%20new%20family%20of%20sulfoxide-functionalized%20NHCs%20was%20created%20by%20a%20simple%20oxidation%20protocol%20from%20thioether-NHC.%20Associated%20with%20Ru%28p-cymene%29%2C%20these%20NHC-SO%20ligands%20allow%20the%20formation%20of%20a%20single%20diastereomer%20in%20the%20form%20of%20an%20enantiomeric%20couple.%20This%20interesting%20selectivity%20is%20discussed%20by%20means%20of%20DFT%20calculations%20and%20experiments.%20image%22%2C%22date%22%3A%222024%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1002%5C%2Fchem.202401390%22%2C%22ISSN%22%3A%220947-6539%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1002%5C%2Fchem.202401390%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222024-08-21T12%3A17%3A01Z%22%7D%7D%2C%7B%22key%22%3A%22AAYNQMFT%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Mechrouk%20et%20al.%22%2C%22parsedDate%22%3A%222024%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EV.%20Mechrouk%2C%20D.%20Bissessar%2C%20J.%20Egly%2C%20J.%20Parmentier%2C%20S.%20Bellemin-Laponnaz%2C%20Synthesis%20and%20Characterization%20of%20Transition%20Metal%20Complexes%20Supported%20by%20Phosphorus%20Ligands%20Obtained%20Using%20Hydrophosphination%20of%20Cyclic%20Internal%20Alkenes.%2C%20Molecules%2029%20%282024%29%203946.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fmolecules29163946%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fmolecules29163946%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Synthesis%20and%20Characterization%20of%20Transition%20Metal%20Complexes%20Supported%20by%20Phosphorus%20Ligands%20Obtained%20Using%20Hydrophosphination%20of%20Cyclic%20Internal%20Alkenes.%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Victoria%22%2C%22lastName%22%3A%22Mechrouk%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Damien%22%2C%22lastName%22%3A%22Bissessar%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Julien%22%2C%22lastName%22%3A%22Egly%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jordan%22%2C%22lastName%22%3A%22Parmentier%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%5D%2C%22abstractNote%22%3A%22The%20design%20and%20study%20of%20rich%2C%20bulky%20phosphorus%20ligands%20is%20a%20key%20area%20of%20research%20for%20homogeneous%20catalysis.%20Here%2C%20we%20describe%20an%20original%20strategy%20using%20a%20hydrophosphination%20reaction%20to%20produce%20phosphines%20of%20interest%20for%20coordination%20chemistry%20and%20homogenous%20catalysis.%20In%20particular%2C%20the%20phosphine%20obtained%20by%20reacting%20diphenylphosphine%20with%20acenaphthylene%20%28ligand%202%29%20gives%20a%20ligand%20that%20adopts%20an%20unusual%20spatial%20geometry.%20The%20coordination%20chemistry%20of%20the%20ligand%20has%20been%20investigated%20with%20Au%28I%29%2C%20Ag%28I%29%2C%20Cu%28I%29%2C%20and%20Pd%28II%29%2C%20for%20which%20a%20complete%20characterization%20could%20be%20made%2C%20particularly%20in%20X-ray%20diffraction%20studies.%20The%20reactivity%20of%20some%20of%20these%20complexes%20has%20been%20demonstrated%2C%20particularly%20in%20Pd-catalyzed%20cross-coupling%20reactions%20and%20Au-catalyzed%20hydroaminations%20and%20in%20the%20hydration%20of%20alkynes.%22%2C%22date%22%3A%222024%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.3390%5C%2Fmolecules29163946%22%2C%22ISSN%22%3A%221420-3049%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.3390%5C%2Fmolecules29163946%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222024-09-13T09%3A16%3A38Z%22%7D%7D%2C%7B%22key%22%3A%22IX7URXAS%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Thierry%20et%20al.%22%2C%22parsedDate%22%3A%222024%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ET.%20Thierry%2C%20Y.%20Geiger%2C%20S.%20Bellemin-Laponnaz%2C%20Divergence%20of%20catalytic%20systems%20in%20the%20zinc-catalysed%20alkylation%20of%20benzaldehyde%20mediated%20by%20chiral%20proline-based%20ligands%2C%20Nature%20Synthesis%20%282024%29.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1038%5C%2Fs44160-024-00491-y%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1038%5C%2Fs44160-024-00491-y%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Divergence%20of%20catalytic%20systems%20in%20the%20zinc-catalysed%20alkylation%20of%20benzaldehyde%20mediated%20by%20chiral%20proline-based%20ligands%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thibault%22%2C%22lastName%22%3A%22Thierry%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yannick%22%2C%22lastName%22%3A%22Geiger%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%5D%2C%22abstractNote%22%3A%22Asymmetric%20catalysis%20has%20expanded%20the%20range%20of%20chiral%20products%20readily%20accessible%20through%20increasingly%20efficient%20synthetic%20catalysts.%20The%20development%20of%20these%20catalysts%20often%20starts%20with%20a%20result%20obtained%20by%20systematic%20screening%20of%20known%20privileged%20chiral%20structures%20and%20assumes%20that%20the%20active%20species%20would%20be%20an%20isolated%20monomolecular%20species.%20Here%20we%20report%20the%20study%20of%20three%20proline-derived%20ligands%2C%20diphenyl-N-methyl-prolinol%2C%20diphenylprolinol%20and%205-%28hydroxydiphenylmethyl%29-2-pyrrolidinone%2C%20in%20the%20zinc-catalysed%20alkylation%20of%20benzaldehyde.%20The%20three%20ligands%20exhibit%20different%20system-level%20behaviour%2C%20characterized%20by%20multiple%20levels%20of%20aggregation%20that%20may%20be%20catalytically%20active%20simultaneously.%20While%20diphenyl-N-methyl-prolinol%20behaves%20as%20expected%20from%20a%20mechanistic%20point%20of%20view%2C%20diphenylprolinol%20shows%20enantiodivergence%20during%20the%20reaction%20due%20to%20an%20asymmetric%20autoinduction%20process.%20With%205-%28hydroxydiphenylmethyl%29-2-pyrrolidinone%2C%20we%20were%20able%20to%20establish%20the%20possibility%20of%20at%20least%20trimeric%20active%20species%20in%20equilibrium%20with%20less%20aggregated%20active%20species.%20Simulations%20using%20a%20mathematical%20model%20confirm%20the%20possibility%20of%20such%20systems-level%20behaviour.%20Parallel%20study%20of%20the%20three%20systems%20reveals%20three%20distinct%20system-level%20behaviours%20that%20are%20central%20to%20the%20efficiency%20of%20the%20catalytic%20reaction.Three%20closely%20related%20proline-based%20ligands%20give%20rise%20to%20different%20catalytic%20systems%20in%20asymmetric%20dialkylzinc%20addition%20reactions.%20Mechanistic%20studies%20reveal%20that%20monomeric%2C%20dimeric%20and%20product-catalyst%20complexes%20and%20aggregates%20larger%20than%20dimers%20are%20all%20catalytically%20active.%22%2C%22date%22%3A%222024%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1038%5C%2Fs44160-024-00491-y%22%2C%22ISSN%22%3A%222731-0582%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1038%5C%2Fs44160-024-00491-y%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222024-06-03T09%3A40%3A05Z%22%7D%7D%2C%7B%22key%22%3A%22EJIQ7DCY%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Thierry%20et%20al.%22%2C%22parsedDate%22%3A%222024%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ET.%20Thierry%2C%20J.%20Frey%2C%20Y.%20Geiger%2C%20S.%20Bellemin-Laponnaz%2C%20Les%20effets%20non%20lin%26%23xE9%3Baires%20en%20catalyse%20asym%26%23xE9%3Btrique%2C%20L%26%23x2019%3Bactualit%26%23xE9%3B%20Chimique%20%282024%29%2040%26%23x2013%3B49.%20%3Ca%20class%3D%27zp-ItemURL%27%20href%3D%27https%3A%5C%2F%5C%2Fnew.societechimiquedefrance.fr%5C%2Fnumero%5C%2Fles-effets-non-lineaires-en-catalyse-asymetrique-p40-n491%5C%2F%27%3Ehttps%3A%5C%2F%5C%2Fnew.societechimiquedefrance.fr%5C%2Fnumero%5C%2Fles-effets-non-lineaires-en-catalyse-asymetrique-p40-n491%5C%2F%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22magazineArticle%22%2C%22title%22%3A%22Les%20effets%20non%20lin%5Cu00e9aires%20en%20catalyse%20asym%5Cu00e9trique%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thibault%22%2C%22lastName%22%3A%22Thierry%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Johanna%22%2C%22lastName%22%3A%22Frey%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yannick%22%2C%22lastName%22%3A%22Geiger%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22St%5Cu00e9phane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222024%22%2C%22language%22%3A%22french%22%2C%22ISSN%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fnew.societechimiquedefrance.fr%5C%2Fnumero%5C%2Fles-effets-non-lineaires-en-catalyse-asymetrique-p40-n491%5C%2F%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222024-06-20T14%3A02%3A25Z%22%7D%7D%2C%7B%22key%22%3A%22ZZEQIZ86%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Thierry%20et%20al.%22%2C%22parsedDate%22%3A%222024%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ET.%20Thierry%2C%20V.%20Giuso%2C%20F.%20Polo%2C%20P.%20Mercandelli%2C%20Y.-T.%20Chen%2C%20C.-H.%20Chang%2C%20M.%20Mauro%2C%20S.%20Bellemin-Laponnaz%2C%20A%20stable%20and%20true-blue%20emissive%20hexacoordinate%20Si%28iv%29%20N-heterocyclic%20carbene%20complex%20and%20its%20use%20in%20organic%20light-emitting%20diodes%2C%20Dalton%20Transactions%2053%20%282024%29%206445%26%23x2013%3B6450.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fd4dt00420e%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fd4dt00420e%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22A%20stable%20and%20true-blue%20emissive%20hexacoordinate%20Si%28iv%29%20N-heterocyclic%20carbene%20complex%20and%20its%20use%20in%20organic%20light-emitting%20diodes%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thibault%22%2C%22lastName%22%3A%22Thierry%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Valerio%22%2C%22lastName%22%3A%22Giuso%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Federico%22%2C%22lastName%22%3A%22Polo%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Pierluigi%22%2C%22lastName%22%3A%22Mercandelli%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yi-Ting%22%2C%22lastName%22%3A%22Chen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Chih-Hao%22%2C%22lastName%22%3A%22Chang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matteo%22%2C%22lastName%22%3A%22Mauro%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%5D%2C%22abstractNote%22%3A%22A%20neutral%20hexacoordinate%20Si%28iv%29%20complex%20containing%20two%20tridentate%20N-heterocyclic%20carbene%20ligands%20is%20synthesised%20and%20characterized%20by%20X-ray%20crystallography%2C%20optical%20spectroscopy%2C%20electrochemistry%20and%20computational%20methods.%20The%20stable%20compound%20exhibits%20remarkable%20deep-blue%20photoluminescence%20particularly%20in%20the%20solid%20state%2C%20which%20enables%20its%20use%20as%20an%20electroluminescent%20material%20in%20organic%20light-emitting%20diodes.%22%2C%22date%22%3A%222024%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1039%5C%2Fd4dt00420e%22%2C%22ISSN%22%3A%221477-9226%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1039%5C%2Fd4dt00420e%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222024-06-20T14%3A03%3A08Z%22%7D%7D%2C%7B%22key%22%3A%22TXMA7N72%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Thierry%20et%20al.%22%2C%22parsedDate%22%3A%222024%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ET.%20Thierry%2C%20Y.%20Geiger%2C%20S.%20Bellemin-Laponnaz%2C%20Correction%3A%20Thierry%20et%20al.%20Observation%20of%20Hyperpositive%20Non-Linear%20Effect%20in%20Asymmetric%20Organozinc%20Alkylation%20in%20Presence%20of%20N-Pyrrolidinyl%20Norephedrine.%20%28Molecules%202022%2C%2027%2C%203780.%29%2C%20Molecules%2029%20%282024%29%204265.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fmolecules29174265%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fmolecules29174265%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Correction%3A%20Thierry%20et%20al.%20Observation%20of%20Hyperpositive%20Non-Linear%20Effect%20in%20Asymmetric%20Organozinc%20Alkylation%20in%20Presence%20of%20N-Pyrrolidinyl%20Norephedrine.%20%28Molecules%202022%2C%2027%2C%203780.%29%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thibault%22%2C%22lastName%22%3A%22Thierry%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yannick%22%2C%22lastName%22%3A%22Geiger%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%5D%2C%22abstractNote%22%3A%22The%20authors%20wish%20to%20make%20the%20following%20correction%20to%20their%20paper%20%5B...%5D.%22%2C%22date%22%3A%222024%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.3390%5C%2Fmolecules29174265%22%2C%22ISSN%22%3A%221420-3049%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.3390%5C%2Fmolecules29174265%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222025-02-13T14%3A22%3A57Z%22%7D%7D%5D%7D
[1]
J.L. Appleton, L. Ballerini, S. Choua, C. Gourlaouen, R. Ruppert, M. Mauro, Cooperative, Close and Remote Steric Effects on the Structural and Optical Properties of Copper(I) Bis-Phenanthroline Complexes, European Journal of Inorganic Chemistry 27 (2024) e202400278.
https://doi.org/10.1002/ejic.202400278 .
[1]
L. Ballerini, W.-M. Zhang, T. Groizard, C. Gourlaouen, F. Polo, A. Jouaiti, H.-C. Su, M. Mauro, Binuclear iridium(iii) complexes for efficient near-infrared light-emitting electrochemical cells with electroluminescence up to 800 nm, Journal of Materials Chemistry C 12 (2024) 12769–12783.
https://doi.org/10.1039/d4tc02040e .
[1]
S. Bellemin-Laponnaz, T. Achard, Recent Progress in Developing Thioether-Containing Ligands for Catalysis Applications, Synthesis-Stuttgart 56 (2024) 1369–1380.
https://doi.org/10.1055/a-2193-4927 .
[1]
C. Dussart, A. Maisse-Francois, S. Bellemin-Laponnaz, Chiral Self-Sorting Process With C2 Symmetric Bisimidazoline Ligands, Chirality 36 (2024) e23720.
https://doi.org/10.1002/chir.23720 .
[1]
C. Dussart, A. Bonnefont, S. Bellemin-Laponnaz, Cu<SUP>I</SUP>/Cu<SUP>II</SUP> Chiral Homoleptic Complexes: Study of Self-Recognition and Self-Discrimination, European Journal of Inorganic Chemistry 27 (2024) e202400527.
https://doi.org/10.1002/ejic.202400527 .
[1]
V. Giuso, C. Gourlaouen, M. Delporte-Pebay, T. Groizard, N. Vanthuyne, J. Crassous, C. Daniel, M. Mauro, Chiroptical activity of benzannulated N-heterocyclic carbene rhenium(i) tricarbonyl halide complexes: towards efficient circularly polarized luminescence emitters, Physical Chemistry Chemical Physics 26 (2024) 4855–4869.
https://doi.org/10.1039/d3cp04300b .
[1]
V. Mechrouk, B. Leforestier, W. Chen, A.I. Poblador-Bahamonde, A. Maisse-Francois, S. Bellemin-Laponnaz, T. Achard, Diastereoselective Synthesis of Sulfoxide-Functionalized N-Heterocyclic Carbene Ruthenium Complexes: An Experimental and Computational Study, Chemistry-a European Journal 30 (2024) e202401390.
https://doi.org/10.1002/chem.202401390 .
[1]
V. Mechrouk, D. Bissessar, J. Egly, J. Parmentier, S. Bellemin-Laponnaz, Synthesis and Characterization of Transition Metal Complexes Supported by Phosphorus Ligands Obtained Using Hydrophosphination of Cyclic Internal Alkenes., Molecules 29 (2024) 3946.
https://doi.org/10.3390/molecules29163946 .
[1]
T. Thierry, Y. Geiger, S. Bellemin-Laponnaz, Divergence of catalytic systems in the zinc-catalysed alkylation of benzaldehyde mediated by chiral proline-based ligands, Nature Synthesis (2024).
https://doi.org/10.1038/s44160-024-00491-y .
[1]
T. Thierry, V. Giuso, F. Polo, P. Mercandelli, Y.-T. Chen, C.-H. Chang, M. Mauro, S. Bellemin-Laponnaz, A stable and true-blue emissive hexacoordinate Si(iv) N-heterocyclic carbene complex and its use in organic light-emitting diodes, Dalton Transactions 53 (2024) 6445–6450.
https://doi.org/10.1039/d4dt00420e .
[1]
T. Thierry, Y. Geiger, S. Bellemin-Laponnaz, Correction: Thierry et al. Observation of Hyperpositive Non-Linear Effect in Asymmetric Organozinc Alkylation in Presence of N-Pyrrolidinyl Norephedrine. (Molecules 2022, 27, 3780.), Molecules 29 (2024) 4265.
https://doi.org/10.3390/molecules29174265 .
1839302
ITCCYZMF
2023
1
surface-science-reports
50
creator
asc
year
10207
https://www.ipcms.fr/plugins/zotpress/
%7B%22status%22%3A%22success%22%2C%22updateneeded%22%3Afalse%2C%22instance%22%3Afalse%2C%22meta%22%3A%7B%22request_last%22%3A0%2C%22request_next%22%3A0%2C%22used_cache%22%3Atrue%7D%2C%22data%22%3A%5B%7B%22key%22%3A%22PED573BR%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Bissessar%20et%20al.%22%2C%22parsedDate%22%3A%222023%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ED.%20Bissessar%2C%20T.%20Thierry%2C%20J.%20Egly%2C%20V.%20Giuso%2C%20T.%20Achard%2C%20P.%20Steffanut%2C%20M.%20Mauro%2C%20S.%20Bellemin-Laponnaz%2C%20Cubane%20Copper%28I%29%20Iodide%20Clusters%20with%20Remotely%20Functionalized%20Phosphine%20Ligands%3A%20Synthesis%2C%20Structural%20Characterization%20and%20Optical%20Properties%2C%20Symmetry-BASEL%2015%20%282023%29%201210.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fsym15061210%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fsym15061210%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Cubane%20Copper%28I%29%20Iodide%20Clusters%20with%20Remotely%20Functionalized%20Phosphine%20Ligands%3A%20Synthesis%2C%20Structural%20Characterization%20and%20Optical%20Properties%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Damien%22%2C%22lastName%22%3A%22Bissessar%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thibault%22%2C%22lastName%22%3A%22Thierry%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Julien%22%2C%22lastName%22%3A%22Egly%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Valerio%22%2C%22lastName%22%3A%22Giuso%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thierry%22%2C%22lastName%22%3A%22Achard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Pascal%22%2C%22lastName%22%3A%22Steffanut%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matteo%22%2C%22lastName%22%3A%22Mauro%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%5D%2C%22abstractNote%22%3A%22We%20present%20here%20the%20synthesis%2C%20chemical%2C%20and%20photophysical%20study%20of%20a%20series%20of%20three%20new%20copper%20halide%20derivatives%2C%20namely%202a-c.%20They%20are%20all%20tetranuclear%20copper-iodide%20clusters%20of%20general%20formula%20%5BCu%28%26%20mu%3B%283%29-I%29P%5D%284%29%20consisting%20of%20a%20cubane-like%20%7BCu4I4%7D%20motif%20and%20P%20%3D%20phosphine.%20They%20differ%20in%20the%20type%20of%20the%20phosphines%20used%20as%20ligands%3A%20a%20monophosphine%20with%20a%20single%20pendant%20ester%20unit%20%28complex%202a%29%2C%20two%20pendant%20ester%20units%20%282b%29%2C%20and%20a%20diphosphine%20containing%20two%20esters%20in%20the%20linker%20%282c%29.%20The%20molecular%20structure%20of%20the%20complexes%20was%20determined%20by%20single-crystal%20X-ray%20diffraction%20analysis.%20All%20the%20investigated%20derivatives%20were%20found%20to%20be%20photo-%20and%20thermally-stable%20luminescent%20species.%20In%20the%20solid%20state%2C%20the%20complexes%20display%20intense%20and%20long-lived%20photoluminescence%20in%20the%20orange%20region%20with%20PLQY%20values%20of%200.43-0.84%20at%20room%20temperature%20associated%20mainly%20with%20a%20%28CC%29-C-3%20excited%20state%20with%20mixed%20%28XMCT%29-X-3%20character.%22%2C%22date%22%3A%222023%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.3390%5C%2Fsym15061210%22%2C%22ISSN%22%3A%222073-8994%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.3390%5C%2Fsym15061210%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222023-08-09T14%3A39%3A34Z%22%7D%7D%2C%7B%22key%22%3A%22VMW452ZB%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22De%20Marco%20et%20al.%22%2C%22parsedDate%22%3A%222023%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ER.%20De%20Marco%2C%20V.%20Giuso%2C%20T.%20Achard%2C%20M.%20Rancan%2C%20M.%20Baron%2C%20L.%20Armelao%2C%20M.%20Mauro%2C%20S.%20Bellemin-Laponnaz%2C%20C.%20Tubaro%2C%20Exploring%20the%20Coordination%20Properties%20of%20Phosphonium-Functionalized%20N-Heterocyclic%20Carbenes%20Towards%20Gold%2C%20European%20Journal%20of%20Inorganic%20Chemistry%20%282023%29%20e202300184.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fejic.202300184%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fejic.202300184%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Exploring%20the%20Coordination%20Properties%20of%20Phosphonium-Functionalized%20N-Heterocyclic%20Carbenes%20Towards%20Gold%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Riccardo%22%2C%22lastName%22%3A%22De%20Marco%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Valerio%22%2C%22lastName%22%3A%22Giuso%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thierry%22%2C%22lastName%22%3A%22Achard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Marzio%22%2C%22lastName%22%3A%22Rancan%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Marco%22%2C%22lastName%22%3A%22Baron%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Lidia%22%2C%22lastName%22%3A%22Armelao%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matteo%22%2C%22lastName%22%3A%22Mauro%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Cristina%22%2C%22lastName%22%3A%22Tubaro%22%7D%5D%2C%22abstractNote%22%3A%22Gold%28I%29%20complexes%20with%20N-heterocyclic%20carbene%20ligands%20functionalized%20with%20a%20pendant%20phosphonium%20moiety%20were%20synthesized%20by%20simple%20procedures.%20In%20particular%2C%20the%20simple%20addition%20of%20LiBr%20salt%20in%20the%20reaction%20media%20allows%20the%20formation%20of%20the%20NHC%20gold%28I%29%20mononuclear%20complexes%2C%20whilst%20in%20the%20absence%20of%20excess%20bromide%20ions%20the%20deprotonation%20of%20the%20methylene%20group%20in%20alpha%20position%20to%20the%20phosphonium%20group%20occurs%2C%20allowing%20the%20isolation%20of%20the%20dinuclear%20complexes%20with%20two%20C%2CC-bridging%20NHC-phosphonium%20ylide%20ligands.%20The%20complexes%20were%20characterized%20in%20solution%20with%20NMR%20spectroscopy%20and%20ESI-MS%20spectrometry%2C%20as%20well%20as%20in%20the%20solid%20state%20by%20means%20of%20single%20crystal%20X-ray%20diffraction%20analysis.%20Mononuclear%20gold%28III%29%20species%20were%20also%20isolated%20by%20Br-2%20oxidative%20addition%20to%20the%20mononuclear%20gold%28I%29%20species%20and%20fully%20characterized.%20Preliminary%20results%20of%20the%20biological%20effects%20on%20MCF7%20cancer%20cells%20are%20also%20reported.%22%2C%22date%22%3A%222023%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1002%5C%2Fejic.202300184%22%2C%22ISSN%22%3A%221434-1948%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1002%5C%2Fejic.202300184%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222023-11-20T14%3A21%3A10Z%22%7D%7D%2C%7B%22key%22%3A%22BYLHAI4S%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Fernandez%20de%20Larrinoa%20et%20al.%22%2C%22parsedDate%22%3A%222023%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EP.%20Fernandez%20de%20Larrinoa%2C%20J.%20Parmentier%2C%20A.%20Kichler%2C%20T.%20Achard%2C%20M.%20Dontenwill%2C%20C.%20Herold-Mende%2C%20S.%20Fournel%2C%20B.%20Frisch%2C%20B.%20Heurtault%2C%20S.%20Bellemin-Laponnaz%2C%20Triphenylphosphonium-functionalized%20N-heterocyclic%20carbene%20platinum%20complexes%20%5B%28NHC-TPP%2B%29Pt%5D%20induce%20cell%20death%20of%20human%20glioblastoma%20cancer%20stem%20cells.%2C%20International%20Journal%20of%20Pharmaceutics%20641%20%282023%29%20123071.%20%3Ca%20class%3D%27zp-ItemURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.ijpharm.2023.123071%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.ijpharm.2023.123071%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Triphenylphosphonium-functionalized%20N-heterocyclic%20carbene%20platinum%20complexes%20%5B%28NHC-TPP%2B%29Pt%5D%20induce%20cell%20death%20of%20human%20glioblastoma%20cancer%20stem%20cells.%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Patricia%22%2C%22lastName%22%3A%22Fernandez%20de%20Larrinoa%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jordan%22%2C%22lastName%22%3A%22Parmentier%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Kichler%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thierry%22%2C%22lastName%22%3A%22Achard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Monique%22%2C%22lastName%22%3A%22Dontenwill%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Christel%22%2C%22lastName%22%3A%22Herold-Mende%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sylvie%22%2C%22lastName%22%3A%22Fournel%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Benoit%22%2C%22lastName%22%3A%22Frisch%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Beatrice%22%2C%22lastName%22%3A%22Heurtault%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%5D%2C%22abstractNote%22%3A%22A%20growing%20body%20of%20experimental%20and%20clinical%20evidence%20suggests%20that%20rare%20cell%20populations%2C%20known%20as%20cancer%20stem%20cells%20%28CSCs%29%2C%20play%20an%20important%20role%20in%20the%20development%20and%20therapeutic%20resistance%20of%20several%20cancers%2C%20including%20glioblastoma.%20Elimination%20of%20these%20cells%20is%20therefore%20of%20paramount%20importance.%20Interestingly%2C%20recent%20results%20have%20shown%20that%20the%20use%20of%20drugs%20that%20specifically%20disrupt%20mitochondria%20or%20induce%20mitochondria-dependent%20apoptosis%20can%20efficiently%20kill%20cancer%20stem%20cells.%20In%20this%20context%2C%20a%20novel%20series%20of%20platinum%28II%29%20complexes%20bearing%20N-heterocyclic%20carbene%20%28NHC%29%20of%20the%20type%20%5B%28NHC%29PtI2%28L%29%5D%20modified%20with%20the%20mitochondria%20targeting%20group%20triphenylphosphonium%20were%20synthesized.%20After%20a%20complete%20characterization%20of%20the%20platinum%20complexes%2C%20the%20cytotoxicity%20against%20two%20different%20cancer%20cell%20lines%2C%20including%20a%20cancer%20stem%20cell%20line%2C%20was%20investigated.%20The%20best%20compound%20reduced%20the%20cell%20viability%20of%20both%20cell%20lines%20by%2050%25%20in%20the%20mM%20range%2C%20with%20an%20approximately%20300-fold%20higher%20anticancer%20activity%20on%20the%20CSC%20line%20compared%20to%20oxaliplatin.%20Finally%2C%20mechanistic%20studies%20showed%20that%20the%20triphenylphosphonium%20functionalized%20platinum%20complexes%20significantly%20altered%20mitochondrial%20function%20and%20also%20induced%20atypical%20cell%20death.%22%2C%22date%22%3A%222023%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.ijpharm.2023.123071%22%2C%22ISSN%22%3A%220378-5173%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.ijpharm.2023.123071%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222023-08-09T10%3A08%3A30Z%22%7D%7D%2C%7B%22key%22%3A%222XXIE2NB%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Giuso%20et%20al.%22%2C%22parsedDate%22%3A%222023%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EV.%20Giuso%2C%20J.%20Yang%2C%20J.%20Fort%26%23xE9%3B%2C%20H.%20Dossmann%2C%20C.%20Daniel%2C%20C.%20Gourlaouen%2C%20M.%20Mauro%2C%20B.%20Bertrand%2C%20Binuclear%20Biphenyl%20Organogold%28III%29%20Complexes%3A%20Synthesis%2C%20Photophysical%20and%20Theoretical%20Investigation%2C%20and%20Anticancer%20Activity%2C%20ChemPlusChem%2088%20%282023%29%20e002300303.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fcplu.202300303%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fcplu.202300303%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Binuclear%20Biphenyl%20Organogold%28III%29%20Complexes%3A%20Synthesis%2C%20Photophysical%20and%20Theoretical%20Investigation%2C%20and%20Anticancer%20Activity%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Valerio%22%2C%22lastName%22%3A%22Giuso%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22JNE%22%2C%22lastName%22%3A%22Yang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22J%22%2C%22lastName%22%3A%22Fort%5Cu00e9%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22H%22%2C%22lastName%22%3A%22Dossmann%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22C%22%2C%22lastName%22%3A%22Daniel%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22C%22%2C%22lastName%22%3A%22Gourlaouen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matteo%22%2C%22lastName%22%3A%22Mauro%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22B%22%2C%22lastName%22%3A%22Bertrand%22%7D%5D%2C%22abstractNote%22%3A%22A%20series%20of%20four%20binuclear%20complexes%20of%20general%20formula%20%5B%28C%3C%5E%3EC%29Au%28Cl%29%28L%3C%5E%3EL%29%28Cl%29Au%28C%3C%5E%3EC%29%5D%2C%20where%20C%3C%5E%3EC%20is%204%2C4%27-diterbutylbiphenyl%20and%20L%3C%5E%3EL%20is%20either%20a%20bridging%20diphosphine%20or%204%2C4%27-bipyridine%2C%20are%20synthetized%20with%2052%20to%2072%20%25%20yield%20and%20structurally%20characterized%20by%20X-ray%20diffraction.%20The%20use%20of%20the%20chelating%201%2C2-diphenylphosphinoethane%20ligand%20in%20a%201%20%3A%202%20%28P%3C%5E%3EP%29%3AAu%20stoichiometry%20leads%20to%20the%20near%20quantitative%20formation%20of%20a%20gold%20double-complex%20salt%20of%20general%20formula%20%5B%28C%3C%5E%3EC%29Au%28P%3C%5E%3EP%29%5D%5B%28C%3C%5E%3EC%3C%5E%3E%29AuCl2%5D.%20The%20compounds%20display%20long-lived%20yellow-green%20phosphorescence%20with%20%26%20lambda%3Bem%20in%20the%20range%20of%20525%20to%20585%20nm%20in%20the%20solid%20state%20with%20photoluminescence%20quantum%20yields%20%28PLQY%29%20up%20to%2010%20%25.%20These%20AuIII%20complexes%20are%20tested%20for%20their%20antiproliferative%20activity%20against%20lung%20adenocarcinoma%20cells%20A549%20and%20results%20show%20that%20compounds%202%20and%205%20are%20the%20most%20promising%20candidates.%20The%20digold%20salt%205%20shows%20anticancer%20activity%20between%2066%20and%20200%20nM%20on%20the%20tested%20cancer%20cell%20lines%2C%20whereas%20derivative%202%20displays%20concentration%20values%20required%20to%20reduce%20by%2050%20%25%20the%20cell%20viability%20%28IC50%29%20between%207%20and%2011%20%26%20mu%3BM.%20Reactivity%20studies%20of%20compound%205%20reveal%20that%20the%20%5B%28C%3C%5E%3EC%29Au%28P%3C%5E%3EP%29%5D%2B%20cation%20is%20stable%20in%20the%20presence%20of%20relevant%20biomolecules%20including%20glutathione%20suggesting%20a%20structural%20mechanism%20of%20action.%5CnFour%20binuclear%20Au%28III%29%20complexes%20have%20been%20synthetized%20using%20non-chelating%20bidentate%20ligands.%20When%20a%20chelating%20ligand%20was%20used%2C%20a%20digold%20salt%20was%20obtained%20resulting%20from%20the%20chelation%20of%20the%20diphosphine%20ligand%20on%20one%20Au%20moiety%20and%20a%20%5B%28C%3C%5E%3EC%29AuCl2%5D-%20anion.%20The%20complexes%20appeared%20strongly%20emissive%20in%20solid%20state%20and%20the%20digold%20salt%20presented%20anticancer%20activities%20in%20the%20nanomolar%20range%20with%20no%20modification%20of%20the%20%5B%28C%3C%5E%3EC%29Au%28P%3C%5E%3EP%29%5D%2B%20in%20the%20presence%20of%20biomolecules.%2Bimage%22%2C%22date%22%3A%222023%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1002%5C%2Fcplu.202300303%22%2C%22ISSN%22%3A%222192-6506%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1002%5C%2Fcplu.202300303%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222023-11-17T14%3A10%3A39Z%22%7D%7D%2C%7B%22key%22%3A%225QGH39PV%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Giuso%20et%20al.%22%2C%22parsedDate%22%3A%222023%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EV.%20Giuso%2C%20E.%20Jouaiti%2C%20C.%20Cebrian%2C%20S.%20Parant-Aury%2C%20N.%20Kyritsakas%2C%20C.%20Gourlaouen%2C%20M.%20Mauro%2C%20Symmetry-Broken%20Charge-Transfer%20Excited%20State%20in%20Homoleptic%20Zinc%28II%29%20Imidazo%5B1%2C2-a%5Dpyridine%20Complexes%2C%20ChemPhotoChem%20%282023%29%20e202300092.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fcptc.202300092%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fcptc.202300092%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Symmetry-Broken%20Charge-Transfer%20Excited%20State%20in%20Homoleptic%20Zinc%28II%29%20Imidazo%5B1%2C2-a%5Dpyridine%20Complexes%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Valerio%22%2C%22lastName%22%3A%22Giuso%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Elise%22%2C%22lastName%22%3A%22Jouaiti%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Cristina%22%2C%22lastName%22%3A%22Cebrian%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sabrina%22%2C%22lastName%22%3A%22Parant-Aury%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nathalie%22%2C%22lastName%22%3A%22Kyritsakas%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Christophe%22%2C%22lastName%22%3A%22Gourlaouen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matteo%22%2C%22lastName%22%3A%22Mauro%22%7D%5D%2C%22abstractNote%22%3A%22A%20series%20of%20four%20homoleptic%20cationic%20Zn-II%20complexes%20%28Zn1-Zn4%29%20coordinated%20by%20two%20substituted%20bis-imidazo%5B1%2C2-a%5Dpyridine%20%28ImPy%29%20ligands%20%28L1-L4%29%20is%20herein%20presented.%20The%20ligands%20are%20functionalized%20with%20either%20electron%20acceptor%20or%20donor%20functional%20groups%20at%20the%20C6%20positions%20of%20the%20two%20ImPy%20moieties.%20In%20DMF%20solution%2C%20all%20of%20the%20complexes%20display%20intense%20near-UV%20to%20blue%20emission%20%28lambda%28em%29%3D379-450%20nm%29%20with%20high%20photoluminescence%20quantum%20yields%20%28PLQY%29%20up%20to%200.50%20and%20short-lived%20excited%20states.%20Additionally%2C%20complex%20Zn4%2C%20containing%20the%20-NPh2%20functionalized%20ligand%2C%20displays%20an%20interesting%20solvatochromic%20behavior.%20The%20absorption%20spectrum%20is%20characterized%20by%20electronic%20transitions%20with%20mainly%20ligand-centered%20%28%28LC%29-L-1%29%20and%20intraligand%20charge-transfer%20%28%28ILCT%29-I-1%29%20character%2C%20which%20involves%20a%20symmetric%20electron%20density%20redistribution%20at%20Franck-Condon%20%28FC%29.%20In%20stark%20contrast%2C%20the%20subsequent%20excited-state%20dynamics%20relaxes%20the%20molecular%20symmetry%20and%20allows%20symmetry-breaking%20hole-electron%20pair%20redistribution%20on%20the%20bichromophoric%20system.%20As%20a%20consequence%2C%20an%20efficient%20radiative%20de-excitation%20process%20takes%20place%20that%20is%20ascribed%20to%20a%20singlet-manifold%20excited%20state%20with%20symmetry-broken%20charge%20transfer%20%28%28SBCT%29-S-1%29%20character%2C%20as%20supported%20by%20an%20in-depth%20photophysical%2C%20electrochemical%20and%20time-dependent%20density%20functional%20theory%20%28TD-DFT%29%20investigation.%20Remarkably%2C%20the%20%28SBCT%29-S-1%20nature%20provides%20structureless%20green%20photoluminescence%20in%20the%20solid%20state%20%28lambda%28em%29%20up%20to%20520%20nm%20for%20Zn4%29%2C%20which%20is%20rather%20uncommon%20for%20Zn-II%20complexes.%22%2C%22date%22%3A%222023%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1002%5C%2Fcptc.202300092%22%2C%22ISSN%22%3A%222367-0932%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1002%5C%2Fcptc.202300092%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222023-11-20T14%3A25%3A42Z%22%7D%7D%2C%7B%22key%22%3A%22SDW8I2UQ%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Jouaiti%20et%20al.%22%2C%22parsedDate%22%3A%222023%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EA.%20Jouaiti%2C%20L.%20Ballerini%2C%20H.-L.%20Shen%2C%20R.%20Viel%2C%20F.%20Polo%2C%20N.%20Kyritsakas%2C%20S.%20Haacke%2C%20Y.-T.%20Huang%2C%20C.-W.%20Lu%2C%20C.%20Gourlaouen%2C%20H.-C.%20Su%2C%20M.%20Mauro%2C%20Binuclear%20Copper%28I%29%20Complexes%20for%20Near-Infrared%20Light-Emitting%20Electrochemical%20Cells.%2C%20Angewandte%20Chemie-International%20Edition%2062%20%282023%29%20e202305569%26%23x2013%3Be202305569.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fanie.202305569%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fanie.202305569%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Binuclear%20Copper%28I%29%20Complexes%20for%20Near-Infrared%20Light-Emitting%20Electrochemical%20Cells.%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Abdelaziz%22%2C%22lastName%22%3A%22Jouaiti%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Lavinia%22%2C%22lastName%22%3A%22Ballerini%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Hsiang-Ling%22%2C%22lastName%22%3A%22Shen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ronan%22%2C%22lastName%22%3A%22Viel%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Federico%22%2C%22lastName%22%3A%22Polo%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nathalie%22%2C%22lastName%22%3A%22Kyritsakas%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stefan%22%2C%22lastName%22%3A%22Haacke%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yu-Ting%22%2C%22lastName%22%3A%22Huang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Chin-Wei%22%2C%22lastName%22%3A%22Lu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Christophe%22%2C%22lastName%22%3A%22Gourlaouen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Hai-Ching%22%2C%22lastName%22%3A%22Su%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matteo%22%2C%22lastName%22%3A%22Mauro%22%7D%5D%2C%22abstractNote%22%3A%22Two%20binuclear%20heteroleptic%20CuI%20complexes%2C%20namely%20Cu-NIR1%20and%20Cu-NIR2%2C%20bearing%20rigid%20chelating%20diphosphines%20and%20pi-conjugated%202%2C5-di%28pyridin-2-yl%29thiazolo%5B5%2C4-d%5Dthiazole%20as%20the%20bis-bidentate%20ligand%20are%20presented.%20The%20proposed%20dinuclearization%20strategy%20yields%20a%20large%20bathochromic%20shift%20of%20the%20emission%20when%20compared%20to%20the%20mononuclear%20counterparts%20%28M1-M2%29%20and%20enables%20shifting%20luminescence%20into%20the%20near-infrared%20%28NIR%29%20region%20in%20both%20solution%20and%20solid%20state%2C%20showing%20emission%20maximum%20at%20ca.%20750%20and%20712%5Cu2005nm%2C%20respectively.%20The%20radiative%20process%20is%20assigned%20to%20an%20excited%20state%20with%20triplet%20metal-to-ligand%20charge%20transfer%20%283%20MLCT%29%20character%20as%20demonstrated%20by%20in-depth%20photophysical%20and%20computational%20investigation.%20Noteworthy%2C%20X-ray%20analysis%20of%20the%20binuclear%20complexes%20unravels%20two%20interligand%20pi-pi-stacking%20interactions%20yielding%20a%20doubly%20locked%20structure%20that%20disfavours%20flattening%20of%20the%20tetrahedral%20coordination%20around%20the%20CuI%20centre%20in%20the%20excited%20state%20and%20maintain%20enhanced%20NIR%20luminescence.%20No%20such%20interaction%20is%20present%20in%20M1-M2.%20These%20findings%20prompt%20the%20successful%20use%20of%20Cu-NIR1%20and%20Cu-NIR2%20in%20NIR%20light-emitting%20electrochemical%20cells%20%28LECs%29%2C%20which%20display%20electroluminescence%20maximum%20up%20to%20756%5Cu2005nm%20and%20peak%20external%20quantum%20efficiency%20%28EQE%29%20of%200.43%25.%20Their%20suitability%20for%20the%20fabrication%20of%20white-emitting%20LECs%20is%20also%20demonstrated.%20To%20the%20best%20of%20our%20knowledge%2C%20these%20are%20the%20first%20examples%20of%20NIR%20electroluminescent%20devices%20based%20on%20earth-abundant%20CuI%20emitters.%22%2C%22date%22%3A%222023%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1002%5C%2Fanie.202305569%22%2C%22ISSN%22%3A%221521-3773%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1002%5C%2Fanie.202305569%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22CHW2VGSR%22%2C%2295EJ8IDX%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222023-11-17T10%3A47%3A56Z%22%7D%7D%2C%7B%22key%22%3A%2273YXF6VS%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Jouaiti%20et%20al.%22%2C%22parsedDate%22%3A%222023%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EA.%20Jouaiti%2C%20D.-C.%20Huang%2C%20V.%20Giuso%2C%20C.%20Cebrian%2C%20P.%20Mercandelli%2C%20K.-H.%20Wang%2C%20C.-H.%20Chang%2C%20M.%20Mauro%2C%20True-%20to%20Sky-Blue%20Emitters%20Bearing%20the%20Thiazolo%5B5%2C4-d%5Dthiazole%20Electron%20Acceptor%20for%20Single%20and%20Tandem%20Organic%20Light-Emitting%20Diodes%2C%20ACS%20Applied%20Electronic%20Materials%205%20%282023%29%202781%26%23x2013%3B2792.%20%3Ca%20class%3D%27zp-ItemURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1021%5C%2Facsaelm.3c00234%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1021%5C%2Facsaelm.3c00234%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22True-%20to%20Sky-Blue%20Emitters%20Bearing%20the%20Thiazolo%5B5%2C4-d%5Dthiazole%20Electron%20Acceptor%20for%20Single%20and%20Tandem%20Organic%20Light-Emitting%20Diodes%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Abdelaziz%22%2C%22lastName%22%3A%22Jouaiti%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Dun-Cheng%22%2C%22lastName%22%3A%22Huang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Valerio%22%2C%22lastName%22%3A%22Giuso%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Cristina%22%2C%22lastName%22%3A%22Cebrian%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Pierluigi%22%2C%22lastName%22%3A%22Mercandelli%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Kuan-Hsun%22%2C%22lastName%22%3A%22Wang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Chih-Hao%22%2C%22lastName%22%3A%22Chang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matteo%22%2C%22lastName%22%3A%22Mauro%22%7D%5D%2C%22abstractNote%22%3A%22A%20series%20of%20emitters%20displaying%20efficient%20photoluminescence%20%28PL%29%20and%20electroluminescence%20%28EL%29%20into%20the%20true%20to%20sky-blue%20regions%20is%20herein%20described%20and%20employed%20as%20electroluminescent%20materials%20for%20the%20fabrication%20of%20efficient%20blue%20organic%20light-emitting%20diodes%20%28OLEDs%29.%20The%20compounds%20possess%20linear%2C%20yet%20a%20twisted%2C%20donor-pi-acceptor-pi-donor%20%28D-pi-A-pi-%20D%29%20architecture%2C%20where%20D%20and%20A%20are%20a%20%28substituted%29%20carbazole%20%28Cz%29%20and%20thiazolo%5B5%2C4-d%5Dthiazole%20%28TzTz%29%20moiety%20as%20the%20electron%20donor%20and%20accepting%20unit%2C%20respectively.%20D%20%3D%209H-carbazol-9-yl%20and%203%2C6-ditert-butyl-9H-carbazol-9-yl%20for%20compounds%20TzTz-PCz2%20and%20TzTzPtbCz2%2C%20respectively.%20In%20dilute%20CH2Cl2%20solutions%2C%20both%20compounds%20display%20intense%20%28PL%20quantum%20yield%20%3D%2060-74%25%29%20and%20short-lived%20%28tau%20%3D%20ca.%200.8-2.0%20ns%29%20luminescence%20arising%20from%20an%20intramolecular%20charge-transfer%20%281ICT%29%20excited%20state%20and%20falling%20into%20the%20sky-blue%20to%20the%20greenish-blue%20region%20%28lambda%20em%20%3D%20448-502%20nm%29.%20Compared%20to%20the%20previously%20reported%20TzTz-TPA%20parental%20emitters%20that%20features%20two%20triphenylamine%20%28TPA%29%20donor%20groups%2C%20the%20highly%20twisted%20nature%20and%20less%20extended%20pi-conjugation%20of%20the%20donor-acceptor%20TzTz-PCz2%20and%20TzTz-PtbCz2%20counterparts%20herein%20presented%20enable%20to%20widen%20the%20HOMO-LUMO%20energy%20gaps%20and%20warrant%20blue%20shifting%20of%20the%20emission.%20Finally%2C%20OLED%20devices%20fabricated%20by%20employing%20the%20herein%20proposed%20emitters%20are%20presented%20as%20well%20with%20both%20single-and%20tandem-device%20architectures.%20The%20so-prepared%20OLEDs%20display%20true-blue%20to%20sky-blue%20EL%20peaking%20at%20lambda%20EL%2Cmax%20%3D%20460%20nm%20%28TzTz-PCz2%29%20and%20467-470%20nm%20%28TzTz-PtbCz2%29%20and%20achieve%20maximum%20external%20quantum%20efficiency%20and%20luminance%20of%2010.2%25%20and%2044%2C851%20cd%20m-2%20%28for%20TzTz-PtbCz2%29%20in%20the%20case%20of%20a%20tandem%20device%2C%20respectively%2C%20with%20a%20mitigated%20roll-off%20efficiency.%20These%20results%20demonstrate%20that%20the%20tandem%20device%20employing%20these%20TzTz%20donor-acceptor%20blue%20emitters%20raises%20the%20feasibility%20of%20fluorescent%20compounds%20to%20compete%20with%20other%20potential%20emitters%20in%20practical%20applications.%22%2C%22date%22%3A%222023%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1021%5C%2Facsaelm.3c00234%22%2C%22ISSN%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1021%5C%2Facsaelm.3c00234%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222025-02-14T13%3A22%3A03Z%22%7D%7D%2C%7B%22key%22%3A%222SWIW256%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22McCartin%20et%20al.%22%2C%22parsedDate%22%3A%222023%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EC.%20McCartin%2C%20J.%20Blumberger%2C%20C.%20Dussouillez%2C%20P.%20Fernandez%20de%20Larrinoa%2C%20M.%20Dontenwill%2C%20C.%20Herold-Mende%2C%20P.%20Lavalle%2C%20B.%20Heurtault%2C%20S.%20Bellemin-Laponnaz%2C%20S.%20Fournel%2C%20A.%20Kichler%2C%20Evaluation%20of%20the%20Cytotoxicity%20of%20Cationic%20Polymers%20on%20Glioblastoma%20Cancer%20Stem%20Cells.%2C%20Journal%20of%20Functional%20Biomaterials%2014%20%282023%29%2017.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fjfb14010017%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fjfb14010017%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Evaluation%20of%20the%20Cytotoxicity%20of%20Cationic%20Polymers%20on%20Glioblastoma%20Cancer%20Stem%20Cells.%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Conor%22%2C%22lastName%22%3A%22McCartin%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Juliette%22%2C%22lastName%22%3A%22Blumberger%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Candice%22%2C%22lastName%22%3A%22Dussouillez%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Patricia%22%2C%22lastName%22%3A%22Fernandez%20de%20Larrinoa%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Monique%22%2C%22lastName%22%3A%22Dontenwill%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Christel%22%2C%22lastName%22%3A%22Herold-Mende%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Philippe%22%2C%22lastName%22%3A%22Lavalle%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Beatrice%22%2C%22lastName%22%3A%22Heurtault%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sylvie%22%2C%22lastName%22%3A%22Fournel%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Kichler%22%7D%5D%2C%22abstractNote%22%3A%22Cationic%20polymers%20such%20as%20polyethylenimine%20%28PEI%29%20have%20found%20a%20pervasive%20place%20in%20laboratories%20across%20the%20world%20as%20gene%20delivery%20agents.%20However%2C%20their%20applications%20are%20not%20limited%20to%20this%20role%2C%20having%20found%20a%20place%20as%20delivery%20agents%20for%20drugs%2C%20in%20complexes%20known%20as%20polymer-drug%20conjugates%20%28PDCs%29.%20Yet%20a%20potentially%20underexplored%20domain%20of%20research%20is%20in%20their%20inherent%20potential%20as%20anti-cancer%20therapeutic%20agents%2C%20which%20has%20been%20indicated%20by%20several%20studies.%20Even%20more%20interesting%20is%20the%20recent%20observation%20that%20certain%20polycations%20may%20present%20a%20significantly%20greater%20toxicity%20towards%20the%20clinically%20important%20cancer%20stem%20cell%20%28CSC%29%20niche%20than%20towards%20more%20differentiated%20bulk%20tumour%20cells.%20These%20cells%2C%20which%20possess%20the%20stem-like%20characteristics%20of%20self-renewal%20and%20differentiation%2C%20are%20highly%20implicated%20in%20cancer%20drug%20resistance%2C%20tumour%20recurrence%20and%20poor%20clinical%20prognosis.%20The%20search%20for%20compounds%20which%20may%20target%20and%20eliminate%20these%20cells%20is%20thus%20of%20great%20research%20interest.%20As%20such%2C%20the%20observation%20in%20our%20previous%20study%20on%20a%20PEI-based%20PDC%20which%20showed%20a%20considerably%20higher%20toxicity%20of%20PEI%20towards%20glioblastoma%20CSCs%20%28GSCs%29%20than%20on%20more%20differentiated%20glioma%20%28U87%29%20cells%20led%20us%20to%20investigate%20other%20cationic%20polymers%20for%20a%20similar%20effect.%20The%20evaluation%20of%20the%20toxicity%20of%20a%20range%20of%20different%20types%20of%20polycations%2C%20and%20an%20investigation%20into%20the%20potential%20source%20of%20GSC%27s%20sensitivity%20to%20such%20compounds%20is%20thus%20described.%22%2C%22date%22%3A%222023%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.3390%5C%2Fjfb14010017%22%2C%22ISSN%22%3A%222079-4983%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.3390%5C%2Fjfb14010017%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222023-12-04T13%3A57%3A25Z%22%7D%7D%2C%7B%22key%22%3A%22D9S37BZP%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Mechrouk%20et%20al.%22%2C%22parsedDate%22%3A%222023%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EV.%20Mechrouk%2C%20A.%20Maisse-Francois%2C%20S.%20Bellemin-Laponnaz%2C%20T.%20Achard%2C%20beta-Alkylation%20through%20Dehydrogenative%20Coupling%20of%20Primary%20Alcohols%20and%20Secondary%20Alcohols%20Catalyzed%20by%20Thioether-Functionalized%20N-Heterocyclic%20Carbene%20Ruthenium%20Complexes%2C%20European%20Journal%20of%20Inorganic%20Chemistry%2026%20%282023%29%20e202300188.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fejic.202300188%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fejic.202300188%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22beta-Alkylation%20through%20Dehydrogenative%20Coupling%20of%20Primary%20Alcohols%20and%20Secondary%20Alcohols%20Catalyzed%20by%20Thioether-Functionalized%20N-Heterocyclic%20Carbene%20Ruthenium%20Complexes%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Victoria%22%2C%22lastName%22%3A%22Mechrouk%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Aline%22%2C%22lastName%22%3A%22Maisse-Francois%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thierry%22%2C%22lastName%22%3A%22Achard%22%7D%5D%2C%22abstractNote%22%3A%22A%20catalytic%20system%20for%20the%20direct%20beta-alkylation%20of%20secondary%20alcohol%20with%20primary%20alcohol%20has%20been%20investigated.%20In%20this%20work%2C%20a%20series%20of%20cationic%20Ru%28II%29%28eta%286%29-p-cymene%29%20complexes%20with%20thioether-functionalized%20N-heterocyclic%20carbene%20ligands%20%28imidazole-based%201a-l%20and%20benzimidazole-based%202a-e%29%20have%20been%20successfully%20synthesized%20and%20evaluated%20as%20catalysts.%20This%20investigation%20shows%20that%20modifications%20in%20the%20ligand%20moiety%20%28thioether%20group%20and%5C%2For%20NHC%20core%29%20have%20a%20strong%20effect%20on%20both%20selectivity%20and%20reactivity.%20Imidazole-based%20complex%201c%2C%20with%20only%201%20mol%25%20of%20catalyst%20loading%2C%20displayed%20the%20best%20catalytic%20activity%20as%20well%20as%20the%20highest%20selectivity%20for%20the%20beta-alcohol%20up%20to%2098%3A%202%20for%20this%20tandem%20borrowing%20hydrogen%5C%2Faldol%20methodology.%20Applied%20to%20a%20wide%20range%20of%20substrates%2C%20beta-alkylated%20secondary%20alcohols%20have%20been%20obtained%20in%20moderate%20yields%2C%20but%20generally%20with%20complete%20conversion%20and%20very%20high%20selectivity.%22%2C%22date%22%3A%222023%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1002%5C%2Fejic.202300188%22%2C%22ISSN%22%3A%221434-1948%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1002%5C%2Fejic.202300188%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222025-02-14T13%3A32%3A35Z%22%7D%7D%2C%7B%22key%22%3A%22AMZDXCVE%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Thibault%20et%20al.%22%2C%22parsedDate%22%3A%222023%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ET.%20Thibault%2C%20M.%20Gurung%2C%20C.%20Leuvrey%2C%20A.%20Boos%2C%20P.%20Ronot%2C%20I.%20El%20Masoudi%2C%20P.%20Hoerner%2C%20S.%20Bellemin-Laponnaz%2C%20Lead-containing%20radiation-attenuating%20sterile%20gloves%20in%20simulated%20use%3A%20Lead%20transfer%20to%20sweat%20as%20an%20unknown%20risk%20to%20users.%2C%20Radiography%2030%20%282023%29%20159%26%23x2013%3B162.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.radi.2023.10.013%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.radi.2023.10.013%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Lead-containing%20radiation-attenuating%20sterile%20gloves%20in%20simulated%20use%3A%20Lead%20transfer%20to%20sweat%20as%20an%20unknown%20risk%20to%20users.%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22T%22%2C%22lastName%22%3A%22Thibault%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22M%22%2C%22lastName%22%3A%22Gurung%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22C%5Cu00e9dric%22%2C%22lastName%22%3A%22Leuvrey%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22A%22%2C%22lastName%22%3A%22Boos%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22P%22%2C%22lastName%22%3A%22Ronot%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22I%22%2C%22lastName%22%3A%22El%20Masoudi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22P%22%2C%22lastName%22%3A%22Hoerner%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22St%5Cu00e9phane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%5D%2C%22abstractNote%22%3A%22BACKGROUND%3A%20Lead%20protective%20gloves%20are%20widely%20used%20to%20attenuate%20scattered%20radiations%20during%20fluoroscopic-guided%20medical%20procedures%2C%20thereby%20reducing%20hand%20exposure%20to%20radiation.AIMS%3A%20To%20determine%20whether%20lead-containing%20gloves%20present%20a%20risk%20of%20metal%20leaching%20onto%20the%20operator%27s%20skin%2C%20particularly%20due%20to%20the%20presence%20of%20sweat.METHODS%3A%20Artificial%20sweat%20of%20varying%20acidity%20was%20introduced%20into%20two%20types%20of%20commercial%20gloves%20containing%20lead.%20The%20level%20of%20lead%20in%20the%20sweat%20was%20then%20assessed%20after%20different%20exposure%20times.%20Electron%20microscopy%20was%20used%20to%20observe%20the%20morphology%20of%20the%20glove%20layers.RESULTS%3A%20Lead%20was%20detected%20in%20artificial%20sweat%20during%20each%20contact%20test%20on%20two%20different%20types%20of%20gloves.%20The%20concentration%20of%20lead%20increased%20with%20the%20acidity%20of%20the%20sweat%2C%20and%20the%20contact%20time.%20Gloves%20with%20a%20protective%20lining%20transferred%20less%20lead%20into%20sweat%2C%20but%20it%20was%20still%20present%20at%20significant%20levels.%20%28i.e.%20few%20milligrams%20of%20lead%20per%20glove%20after%20one%20hour%20contact%29.CONCLUSIONS%3A%20Fluoroscopy%20operators%20should%20be%20aware%20of%20the%20risk%20of%20leaching%20of%20lead%20ions%20when%20using%20lead%20gloves%20under%20intensive%20conditions%2C%20although%20the%20potential%20harmfulness%20of%20lead%20ions%20leached%20into%20the%20glove%20remains%20essentially%20unknown.%22%2C%22date%22%3A%222023%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.radi.2023.10.013%22%2C%22ISSN%22%3A%221532-2831%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1016%5C%2Fj.radi.2023.10.013%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%226IWM732K%22%2C%22CF4ZI7HM%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222024-03-05T10%3A07%3A01Z%22%7D%7D%2C%7B%22key%22%3A%22QT75DR46%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Vila%20et%20al.%22%2C%22parsedDate%22%3A%222023%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EJ.%20Vila%2C%20M.%20Sol%26%23xE0%3B%2C%20T.%20Achard%2C%20S.%20Bellemin-Laponnaz%2C%20A.%20Pla-Quintana%2C%20A.%20Roglans%2C%20Rh%28I%29%20Complexes%20with%20Hemilabile%20Thioether-Functionalized%20NHC%20Ligands%20as%20Catalysts%20for%20%5B2%20%2B%202%20%2B%202%5D%20Cycloaddition%20of%201%2C5-Bisallenes%20and%20Alkynes%2C%20ACS%20Catal.%2013%20%282023%29%203201%26%23x2013%3B3210.%20%3Ca%20class%3D%27zp-ItemURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1021%5C%2Facscatal.2c05790%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1021%5C%2Facscatal.2c05790%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Rh%28I%29%20Complexes%20with%20Hemilabile%20Thioether-Functionalized%20NHC%20Ligands%20as%20Catalysts%20for%20%5B2%20%2B%202%20%2B%202%5D%20Cycloaddition%20of%201%2C5-Bisallenes%20and%20Alkynes%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jordi%22%2C%22lastName%22%3A%22Vila%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Miquel%22%2C%22lastName%22%3A%22Sol%5Cu00e0%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thierry%22%2C%22lastName%22%3A%22Achard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22St%5Cu00e9phane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Anna%22%2C%22lastName%22%3A%22Pla-Quintana%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Anna%22%2C%22lastName%22%3A%22Roglans%22%7D%5D%2C%22abstractNote%22%3A%22The%20%5B2%20%2B%202%20%2B%202%5D%20cycloaddition%20of%201%2C5-bisallenes%20and%20alkynes%20under%20the%20catalysis%20of%20Rh%28I%29%20with%20hemilabile%20thioether-functionalized%20N-heterocyclic%20carbene%20ligands%20is%20described.%20This%20protocol%20effectively%20provides%20an%20entry%20to%20different%20trans-5%2C6-fused%20bicyclic%20systems%20with%20two%20exocyclic%20double%20bonds%20in%20the%20cyclohexene%20ring.%20The%20process%20is%20totally%20chemoselective%20with%20the%20two%20internal%20double%20bonds%20of%20the%201%2C5-bisallenes%20being%20involved%20in%20the%20cycloaddition.%20The%20complete%20mechanism%20of%20this%20transformation%20as%20well%20as%20the%20preference%20for%20the%20trans-fusion%20over%20the%20cis-fusion%20has%20been%20rationalized%20by%20density%20functional%20theory%20calculations.%20The%20reaction%20follows%20a%20typical%20%5B2%20%2B%202%20%2B%202%5D%20cycloaddition%20mechanism.%20The%20oxidative%20addition%20takes%20place%20between%20the%20alkyne%20and%20one%20of%20the%20allenes%20and%20it%20is%20when%20the%20second%20allene%20is%20inserted%20into%20the%20rhodacyclopentene%20that%20the%20trans-fusion%20is%20generated.%20Remarkably%2C%20the%20hemilabile%20character%20of%20the%20sulfur%20atom%20in%20the%20N-heterocyclic%20carbene%20ligand%20modulates%20the%20electron%20density%20in%20key%20intermediates%2C%20facilitating%20the%20overall%20transformation.%22%2C%22date%22%3A%222023%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1021%5C%2Facscatal.2c05790%22%2C%22ISSN%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1021%5C%2Facscatal.2c05790%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222023-02-22T07%3A40%3A17Z%22%7D%7D%2C%7B%22key%22%3A%22RELV6ZXX%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Yang%20et%20al.%22%2C%22parsedDate%22%3A%222023%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EJ.%20Yang%2C%20V.%20Giuso%2C%20M.-C.%20Hou%2C%20E.%20Remadna%2C%20J.%20Forte%2C%20H.-C.%20Su%2C%20C.%20Gourlaouen%2C%20M.%20Mauro%2C%20B.%20Bertrand%2C%20Biphenyl%20Au%28III%29%20Complexes%20with%20Phosphine%20Ancillary%20Ligands%3A%20Synthesis%2C%20Optical%20Properties%2C%20and%20Electroluminescence%20in%20Light-Emitting%20Electrochemical%20Cells.%2C%20Inorganic%20Chemistry%2062%20%282023%29%204903%26%23x2013%3B4921.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1021%5C%2Facs.inorgchem.2c04293%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1021%5C%2Facs.inorgchem.2c04293%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Biphenyl%20Au%28III%29%20Complexes%20with%20Phosphine%20Ancillary%20Ligands%3A%20Synthesis%2C%20Optical%20Properties%2C%20and%20Electroluminescence%20in%20Light-Emitting%20Electrochemical%20Cells.%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jeannine%22%2C%22lastName%22%3A%22Yang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Valerio%22%2C%22lastName%22%3A%22Giuso%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Min-Chih%22%2C%22lastName%22%3A%22Hou%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Edwyn%22%2C%22lastName%22%3A%22Remadna%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jeremy%22%2C%22lastName%22%3A%22Forte%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Hai-Ching%22%2C%22lastName%22%3A%22Su%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Christophe%22%2C%22lastName%22%3A%22Gourlaouen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matteo%22%2C%22lastName%22%3A%22Mauro%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Benoit%22%2C%22lastName%22%3A%22Bertrand%22%7D%5D%2C%22abstractNote%22%3A%22A%20series%20of%20ten%20cationic%20complexes%20of%20the%20general%20formula%20%5B%28C%5EC%29Au%28P%5EP%29%5DX%2C%20where%20C%5EC%20%3D%204%2C4%27-di-tert-butyl-1%2C1%27-biphenyl%2C%20P%5EP%20is%20a%20diphosphine%20ligand%2C%20and%20X%20is%20a%20noncoordinating%20counteranion%2C%20have%20been%20synthesized%20and%20fully%20characterized%20by%20means%20of%20chemical%20and%20X-ray%20structural%20methods.%20All%20the%20complexes%20display%20a%20remarkable%20switch-on%20of%20the%20emission%20properties%20when%20going%20from%20a%20fluid%20solution%20to%20a%20solid%20state.%20In%20the%20latter%2C%20long-lived%20emission%20with%20lifetime%20tau%20%3D%201.8-83.0%20mus%20and%20maximum%20in%20the%20green-yellow%20region%20is%20achieved%20with%20moderate%20to%20high%20photoluminescence%20quantum%20yield%20%28PLQY%29.%20This%20emission%20is%20ascribed%20to%20an%20excited%20state%20with%20a%20mainly%20triplet%20ligand-centered%20%283LC%29%20nature.%20This%20effect%20strongly%20indicates%20that%20rigidification%20of%20the%20environment%20helps%20to%20suppress%20nonradiative%20decay%2C%20which%20is%20mainly%20attributed%20to%20the%20large%20molecular%20distortion%20in%20the%20excited%20state%2C%20as%20supported%20by%20density%20functional%20theory%20%28DFT%29%20and%20time-dependent%20DFT%20%28TD-DFT%29%20computation.%20In%20addition%2C%20quenching%20intermolecular%20interactions%20of%20the%20emitter%20are%20avoided%20thanks%20to%20the%20steric%20hindrance%20of%20the%20substituents.%20Emissive%20properties%20are%20therefore%20restored%20efficiently.%20The%20influence%20of%20both%20diphosphine%20and%20anion%20has%20been%20investigated%20and%20rationalized%20as%20well.%20Using%20two%20complexes%20as%20examples%20and%20owing%20to%20their%20enhanced%20optical%20properties%20in%20the%20solid%20state%2C%20the%20first%20proof-of-concept%20of%20the%20use%20of%20gold%28III%29%20complexes%20as%20electroactive%20materials%20for%20the%20fabrication%20of%20light-emitting%20electrochemical%20cell%20%28LEC%29%20devices%20is%20herein%20demonstrated.%20The%20LECs%20achieve%20peak%20external%20quantum%20efficiency%2C%20current%20efficiency%2C%20and%20power%20efficiency%20up%20to%20ca.%201%25%2C%202.6%20cd%20A-1%2C%20and%201.1%20lm%20W-1%20for%20complex%201PF6%20and%200.9%25%2C%202.5%20cd%20A-1%2C%20and%200.7%20lm%20W-1%20for%20complex%203%2C%20showing%20the%20potential%20use%20of%20these%20novel%20emitters%20as%20electroactive%20compounds%20in%20LEC%20devices.%22%2C%22date%22%3A%222023%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1021%5C%2Facs.inorgchem.2c04293%22%2C%22ISSN%22%3A%221520-510X%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1021%5C%2Facs.inorgchem.2c04293%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222023-05-31T13%3A25%3A47Z%22%7D%7D%5D%7D
[1]
D. Bissessar, T. Thierry, J. Egly, V. Giuso, T. Achard, P. Steffanut, M. Mauro, S. Bellemin-Laponnaz, Cubane Copper(I) Iodide Clusters with Remotely Functionalized Phosphine Ligands: Synthesis, Structural Characterization and Optical Properties, Symmetry-BASEL 15 (2023) 1210.
https://doi.org/10.3390/sym15061210 .
[1]
R. De Marco, V. Giuso, T. Achard, M. Rancan, M. Baron, L. Armelao, M. Mauro, S. Bellemin-Laponnaz, C. Tubaro, Exploring the Coordination Properties of Phosphonium-Functionalized N-Heterocyclic Carbenes Towards Gold, European Journal of Inorganic Chemistry (2023) e202300184.
https://doi.org/10.1002/ejic.202300184 .
[1]
P. Fernandez de Larrinoa, J. Parmentier, A. Kichler, T. Achard, M. Dontenwill, C. Herold-Mende, S. Fournel, B. Frisch, B. Heurtault, S. Bellemin-Laponnaz, Triphenylphosphonium-functionalized N-heterocyclic carbene platinum complexes [(NHC-TPP+)Pt] induce cell death of human glioblastoma cancer stem cells., International Journal of Pharmaceutics 641 (2023) 123071.
https://doi.org/10.1016/j.ijpharm.2023.123071 .
[1]
V. Giuso, J. Yang, J. Forté, H. Dossmann, C. Daniel, C. Gourlaouen, M. Mauro, B. Bertrand, Binuclear Biphenyl Organogold(III) Complexes: Synthesis, Photophysical and Theoretical Investigation, and Anticancer Activity, ChemPlusChem 88 (2023) e002300303.
https://doi.org/10.1002/cplu.202300303 .
[1]
V. Giuso, E. Jouaiti, C. Cebrian, S. Parant-Aury, N. Kyritsakas, C. Gourlaouen, M. Mauro, Symmetry-Broken Charge-Transfer Excited State in Homoleptic Zinc(II) Imidazo[1,2-a]pyridine Complexes, ChemPhotoChem (2023) e202300092.
https://doi.org/10.1002/cptc.202300092 .
[1]
A. Jouaiti, L. Ballerini, H.-L. Shen, R. Viel, F. Polo, N. Kyritsakas, S. Haacke, Y.-T. Huang, C.-W. Lu, C. Gourlaouen, H.-C. Su, M. Mauro, Binuclear Copper(I) Complexes for Near-Infrared Light-Emitting Electrochemical Cells., Angewandte Chemie-International Edition 62 (2023) e202305569–e202305569.
https://doi.org/10.1002/anie.202305569 .
[1]
A. Jouaiti, D.-C. Huang, V. Giuso, C. Cebrian, P. Mercandelli, K.-H. Wang, C.-H. Chang, M. Mauro, True- to Sky-Blue Emitters Bearing the Thiazolo[5,4-d]thiazole Electron Acceptor for Single and Tandem Organic Light-Emitting Diodes, ACS Applied Electronic Materials 5 (2023) 2781–2792.
https://doi.org/10.1021/acsaelm.3c00234 .
[1]
C. McCartin, J. Blumberger, C. Dussouillez, P. Fernandez de Larrinoa, M. Dontenwill, C. Herold-Mende, P. Lavalle, B. Heurtault, S. Bellemin-Laponnaz, S. Fournel, A. Kichler, Evaluation of the Cytotoxicity of Cationic Polymers on Glioblastoma Cancer Stem Cells., Journal of Functional Biomaterials 14 (2023) 17.
https://doi.org/10.3390/jfb14010017 .
[1]
V. Mechrouk, A. Maisse-Francois, S. Bellemin-Laponnaz, T. Achard, beta-Alkylation through Dehydrogenative Coupling of Primary Alcohols and Secondary Alcohols Catalyzed by Thioether-Functionalized N-Heterocyclic Carbene Ruthenium Complexes, European Journal of Inorganic Chemistry 26 (2023) e202300188.
https://doi.org/10.1002/ejic.202300188 .
[1]
T. Thibault, M. Gurung, C. Leuvrey, A. Boos, P. Ronot, I. El Masoudi, P. Hoerner, S. Bellemin-Laponnaz, Lead-containing radiation-attenuating sterile gloves in simulated use: Lead transfer to sweat as an unknown risk to users., Radiography 30 (2023) 159–162.
https://doi.org/10.1016/j.radi.2023.10.013 .
[1]
J. Vila, M. Solà, T. Achard, S. Bellemin-Laponnaz, A. Pla-Quintana, A. Roglans, Rh(I) Complexes with Hemilabile Thioether-Functionalized NHC Ligands as Catalysts for [2 + 2 + 2] Cycloaddition of 1,5-Bisallenes and Alkynes, ACS Catal. 13 (2023) 3201–3210.
https://doi.org/10.1021/acscatal.2c05790 .
[1]
J. Yang, V. Giuso, M.-C. Hou, E. Remadna, J. Forte, H.-C. Su, C. Gourlaouen, M. Mauro, B. Bertrand, Biphenyl Au(III) Complexes with Phosphine Ancillary Ligands: Synthesis, Optical Properties, and Electroluminescence in Light-Emitting Electrochemical Cells., Inorganic Chemistry 62 (2023) 4903–4921.
https://doi.org/10.1021/acs.inorgchem.2c04293 .
1839302
ITCCYZMF
2022
1
surface-science-reports
50
creator
asc
year
10207
https://www.ipcms.fr/plugins/zotpress/
%7B%22status%22%3A%22success%22%2C%22updateneeded%22%3Afalse%2C%22instance%22%3Afalse%2C%22meta%22%3A%7B%22request_last%22%3A0%2C%22request_next%22%3A0%2C%22used_cache%22%3Atrue%7D%2C%22data%22%3A%5B%7B%22key%22%3A%22E6FVAU35%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Bissessar%20et%20al.%22%2C%22parsedDate%22%3A%222022%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ED.%20Bissessar%2C%20J.%20Egly%2C%20T.%20Achard%2C%20P.%20Steffanut%2C%20M.%20Mauro%2C%20S.%20Bellemin-Laponnaz%2C%20A%20Stable%20and%20Photoreactive%20Copper-Iodide%20Cubane%20Suitable%20for%20Direct%20Post-Functionalization%2C%20European%20Journal%20of%20Inorganic%20Chemistry%20%282022%29%20e202200101.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fejic.202200101%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fejic.202200101%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22A%20Stable%20and%20Photoreactive%20Copper-Iodide%20Cubane%20Suitable%20for%20Direct%20Post-Functionalization%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Damien%22%2C%22lastName%22%3A%22Bissessar%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Julien%22%2C%22lastName%22%3A%22Egly%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thierry%22%2C%22lastName%22%3A%22Achard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Pascal%22%2C%22lastName%22%3A%22Steffanut%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matteo%22%2C%22lastName%22%3A%22Mauro%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%5D%2C%22abstractNote%22%3A%22Tetranuclear%20copper-iodide%20cluster%20consisting%20of%20a%20%7BCu4I4%7D%20cubane%20and%20diphenylphosphine%20ligand%20has%20been%20found%20as%20a%20highly%20stable%20precursor%20for%20further%20post-functionalization%20through%20hydrophosphination%20reaction.%20UV%20irradiation%20of%20the%20cluster%20in%20the%20presence%20of%20various%20alkenes%20allows%20direct%20functionalization%20of%20the%20latter%2C%20giving%20access%20to%20a%20wide%20range%20of%20copper%20cubane%20complexes%2C%20a%20source%20of%20molecular%20diversity%20for%20luminescent%20materials.%20Such%20a%20strategy%20provided%20access%20to%20the%20first%20example%20of%20water-soluble%20luminescent%20cubane%20copper%20iodide.%22%2C%22date%22%3A%222022%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1002%5C%2Fejic.202200101%22%2C%22ISSN%22%3A%221434-1948%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1002%5C%2Fejic.202200101%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222022-07-19T11%3A20%3A23Z%22%7D%7D%2C%7B%22key%22%3A%22PXGV5P63%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Bonfiglio%20et%20al.%22%2C%22parsedDate%22%3A%222022%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EA.%20Bonfiglio%2C%20P.-W.%20Hsiao%2C%20Y.%20Chen%2C%20C.%20Gourlaouen%2C%20Q.%20Marchand%2C%20V.%20Cesar%2C%20S.%20Bellemin-Laponnaz%2C%20Y.-X.%20Wang%2C%20C.-W.%20Lu%2C%20C.%20Daniel%2C%20F.%20Polo%2C%20H.-C.%20Su%2C%20M.%20Mauro%2C%20Highly%20Emissive%20Red%20Heterobimetallic%20Ir-III%5C%2FM-I%20%28M-I%20%3D%20Cu-I%20and%20Au-I%29%20Complexes%20for%20Efficient%20Light-Emitting%20Electrochemical%20Cells%2C%20Chemistry%20of%20Materials%2034%20%282022%29%201756%26%23x2013%3B1769.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1021%5C%2Facs.chemmater.1c03972%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1021%5C%2Facs.chemmater.1c03972%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Highly%20Emissive%20Red%20Heterobimetallic%20Ir-III%5C%2FM-I%20%28M-I%20%3D%20Cu-I%20and%20Au-I%29%20Complexes%20for%20Efficient%20Light-Emitting%20Electrochemical%20Cells%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Anna%22%2C%22lastName%22%3A%22Bonfiglio%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Pei-Wan%22%2C%22lastName%22%3A%22Hsiao%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yin%22%2C%22lastName%22%3A%22Chen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Christophe%22%2C%22lastName%22%3A%22Gourlaouen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Quentin%22%2C%22lastName%22%3A%22Marchand%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Vincent%22%2C%22lastName%22%3A%22Cesar%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yun-Xin%22%2C%22lastName%22%3A%22Wang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Chin-Wei%22%2C%22lastName%22%3A%22Lu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Chantal%22%2C%22lastName%22%3A%22Daniel%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Federico%22%2C%22lastName%22%3A%22Polo%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Hai-Ching%22%2C%22lastName%22%3A%22Su%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matteo%22%2C%22lastName%22%3A%22Mauro%22%7D%5D%2C%22abstractNote%22%3A%22A%20class%20of%20four%20bright%20heterobimetallic%20emitters%20is%20presented%2C%20which%20features%20both%20a%20chromophoric%20%5BIr%28C%20boolean%20AND%20N%29%282%29%5D%20center%20and%20a%20positively%20charged%2C%20linear%20bis-NHC%20M%28I%29%20ancillary%20moiety%20bridged%20through%20a%20Janus-type%20ligand.%20An%20in-depth%20investigation%20of%20their%20optical%20and%20electrochemical%20properties%20is%20also%20reported%2C%20which%20are%20further%20elucidated%20by%20means%20of%20time-dependent%20density%20functional%20theory%20including%20spin-orbital%20coupling%20effects.%20All%20complexes%20display%20efficient%2C%20vibrant%20red%20phosphorescence%20with%20a%20higher%20quantum%20yield%20and%20a%20faster%20radiative%20rate%20constant%20compared%20to%20the%20mononuclear%20parental%20complexes.%20This%20effect%20was%20elucidated%20in%20terms%20of%20better%20S-T%20excited-state%20mixing%20as%20well%20as%20increased%20rigidity%20favored%20by%20the%20multimetallic%20architecture.%20Finally%2C%20their%20electroluminescence%20performances%20are%20investigated%20by%20using%20these%20bimetallic%20complexes%20as%20electroactive%20materials%20in%20light-emitting%20electrochemical%20cells%2C%20achieving%20external%20quantum%20efficiency%20values%20up%20to%206%25%20and%20resulting%20in%20among%20the%20most%20efficient%20ones%20for%20red%20emitters.%20This%20result%20is%20also%20attributable%20to%20the%20charge-neutral%20nature%20of%20an%20emitting%20Ir%20complex%20bearing%20a%20charged%2C%20wider%20energy%20gap%2C%20metal%20complex%20as%20an%20ancillary%20moiety.%22%2C%22date%22%3A%222022%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1021%5C%2Facs.chemmater.1c03972%22%2C%22ISSN%22%3A%220897-4756%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1021%5C%2Facs.chemmater.1c03972%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222022-07-21T09%3A01%3A14Z%22%7D%7D%2C%7B%22key%22%3A%22HFVLZX4P%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Egly%20et%20al.%22%2C%22parsedDate%22%3A%222022%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EJ.%20Egly%2C%20W.%20Chen%2C%20A.%20Maisse-Francois%2C%20S.%20Bellemin-Laponnaz%2C%20T.%20Achard%2C%20Half-Sandwich%20Ruthenium%20Complexes%20Bearing%20Hemilabile%20kappa%282%29-%28C%2CS%29-Thioether-Functionalized%20NHC%20Ligands%3A%20Application%20to%20Amide%20Synthesis%20from%20Alcohol%20and%20Amine%2C%20European%20Journal%20of%20Inorganic%20Chemistry%20%282022%29%20e202101033.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fejic.202101033%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fejic.202101033%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Half-Sandwich%20Ruthenium%20Complexes%20Bearing%20Hemilabile%20kappa%282%29-%28C%2CS%29-Thioether-Functionalized%20NHC%20Ligands%3A%20Application%20to%20Amide%20Synthesis%20from%20Alcohol%20and%20Amine%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Julien%22%2C%22lastName%22%3A%22Egly%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Weighang%22%2C%22lastName%22%3A%22Chen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Aline%22%2C%22lastName%22%3A%22Maisse-Francois%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thierry%22%2C%22lastName%22%3A%22Achard%22%7D%5D%2C%22abstractNote%22%3A%22Amide%20synthesis%20is%20one%20of%20the%20most%20crucial%20transformations%20in%20chemistry%20and%20biology.%20Among%20various%20catalytic%20systems%2C%20N-heterocyclic%20carbene%20%28NHC%29-based%20ruthenium%20%28Ru%29%20catalyst%20systems%20have%20been%20proven%20to%20be%20active%20for%20direct%20synthesis%20of%20amides%20by%20sustainable%20acceptorless%20dehydrogenative%20Coupling%20of%20primary%20alcohols%20with%20amines.%20Most%20often%2C%20these%20catalytic%20systems%20usually%20use%20monodentate%20NHC%20and%20thus%20require%20an%20additional%20ligand%20to%20obtain%20high%20reactivity%20and%20selectivity.%20In%20this%20work%2C%20a%20series%20of%20cationic%20Ru%28II%29%28eta%286%29-p-cymene%29%20complexes%20with%20thioether-functionalized%20N-heterocyclic%20carbene%20ligands%20%28imidazole%20and%20benzimidazole-based%29%20have%20been%20prepared%20and%20fully%20characterized.%20These%20complexes%20have%20then%20been%20used%20in%20the%20amidation%20reaction%20and%20the%20most%20promising%20one%20%28i.%20e.%203%20c%29%20has%20been%20applied%20on%20a%20large%20range%20of%20substrates.%20High%20conversions%20albeit%20with%20moderate%20yields%20have%20generally%20been%20obtained.%22%2C%22date%22%3A%222022%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1002%5C%2Fejic.202101033%22%2C%22ISSN%22%3A%221434-1948%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1002%5C%2Fejic.202101033%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222025-04-15T07%3A37%3A43Z%22%7D%7D%2C%7B%22key%22%3A%223G5HB369%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Geiger%20and%20Bellemin-Laponnaz%22%2C%22parsedDate%22%3A%222022%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EY.%20Geiger%2C%20S.%20Bellemin-Laponnaz%2C%20Non-Linear%20Effects%20in%20Asymmetric%20Catalysis%3A%20Impact%20of%20Catalyst%20Precipitation%2C%20ChemCatChem%20%282022%29%20e202200165.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fcctc.202200165%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fcctc.202200165%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Non-Linear%20Effects%20in%20Asymmetric%20Catalysis%3A%20Impact%20of%20Catalyst%20Precipitation%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yannick%22%2C%22lastName%22%3A%22Geiger%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22St%5Cu00e9phane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%5D%2C%22abstractNote%22%3A%22Non-linear%20effects%20between%20the%20enantiomeric%20excesses%20of%20both%20the%20ligand%20and%20the%20product%20are%20ubiquitous%20phenomena%20in%20asymmetric%20catalysis%2C%20allowing%20asymmetric%20amplification%20%28or%20depletion%29%20and%20are%20widely%20used%20tools%20for%20mechanistic%20investigations.%20Non-linear%20effects%20are%20caused%20by%20catalyst%20aggregation%3B%20however%2C%20the%20effect%20of%20catalyst%20precipitation%20on%20NLEs%20has%20not%20been%20systematically%20investigated%20to%20date%2C%20except%20in%20special%20cases%20such%20as%20ternary%20phase%20systems.%20In%20this%20article%2C%20we%20show%20through%20simulations%20and%20with%20several%20literature%20cases%20at%20hand%20how%20precipitation%20affects%20shape%20and%20amplitude%20of%20NLE%20curves.%20The%20limit%20of%20solubility%20of%20the%20homo-%20or%20heterochiral%20dimeric%20species%20causes%20broken-shaped%20NLE%20curves%20or%20very%20pronounced%20NLEs%20even%20though%20the%20equilibria%20between%20the%20different%20species%20in%20solution%20are%20not%20favorable%2C%20at%20first%20sight.%20Peculiar%20features%20such%20as%20horizontal%20segments%2C%20inverse%20S-shaped%20curves%20and%20a%20strong%20effect%20of%20total%20catalyst%20concentration%20are%20also%20observed.%20Overall%2C%20this%20study%20allows%20to%20get%20a%20better%20understanding%20of%20chiral%20catalytic%20systems%20and%20gives%20an%20outlook%20at%20other%20types%20of%20phase%20separation%20leading%20to%20NLEs.%22%2C%22date%22%3A%222022%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1002%5C%2Fcctc.202200165%22%2C%22ISSN%22%3A%221867-3880%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1002%5C%2Fcctc.202200165%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222022-04-20T09%3A50%3A41Z%22%7D%7D%2C%7B%22key%22%3A%22K5WU8B5I%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Jouaiti%20et%20al.%22%2C%22parsedDate%22%3A%222022%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EE.%20Jouaiti%2C%20V.%20Giuso%2C%20D.%20Cianfarani%2C%20N.%20Kyritsakas%2C%20C.%20Gourlaouen%2C%20M.%20Mauro%2C%20Bright%20V-Shaped%20bis-Imidazo%5B1%2C2-a%5Dpyridine%20Fluorophores%20with%20Near-UV%20to%20Deep-Blue%20Emission.%2C%20Chemistry%2C%20an%20Asian%20Journal%20%282022%29%20e202200903%26%23x2013%3Be202200903.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fasia.202200903%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fasia.202200903%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Bright%20V-Shaped%20bis-Imidazo%5B1%2C2-a%5Dpyridine%20Fluorophores%20with%20Near-UV%20to%20Deep-Blue%20Emission.%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Elise%22%2C%22lastName%22%3A%22Jouaiti%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Valerio%22%2C%22lastName%22%3A%22Giuso%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Damien%22%2C%22lastName%22%3A%22Cianfarani%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nathalie%22%2C%22lastName%22%3A%22Kyritsakas%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Christophe%22%2C%22lastName%22%3A%22Gourlaouen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matteo%22%2C%22lastName%22%3A%22Mauro%22%7D%5D%2C%22abstractNote%22%3A%22Ten%20novel%20small-molecule%20fluorophores%20containing%20two%20electron-accepting%20imidazo%5B1%2C2-a%5Dpyridine%20%28ImPy%29%20units%20are%20presented.%20Each%20ImPy%20core%20is%20functionalized%20at%20its%20C6%20position%20with%20groups%20featuring%20either%20electron%20accepting%20%28A%29%20or%20donating%20%28D%29%20properties%2C%20thus%20providing%20emitters%20with%20general%20structure%20X-ImPy-Y-ImPy-X%20%28X%3Deither%20A%20or%20D%3B%20Y%3Dphenyl%20or%20pyridine%29.%20The%20molecules%20bear%20either%20a%20phenyl%20%28series%204%29%20or%20a%20pyridine%20%28series%205%29%20pi%20bridge%20that%20connects%20the%20two%20ImPys%20via%20meta%20%28phenyl%29%20or%202%2C6-%20%28pyridine%29%20positions%2C%20yielding%20an%20overall%20V-shaped%20architecture.%20The%20final%20compounds%20are%20synthetized%20straightforwardly%20by%20condensation%20between%20substituted%202-aminopyridines%20and%20alpha-halocarbonyl%20derivatives.%20All%20the%20compounds%20display%20intense%20photoluminescence%20with%20quantum%20yield%20%28PLQY%29%20in%20the%20range%20of%200.17-0.51.%20Remarkably%2C%20substituent%20effect%20enables%20tuning%20the%20emission%20from%20near-UV%20to%20%28deep-%29blue%20region%20while%20keeping%20Commission%20Internationale%20de%20l%27Eclairage%20%28CIE%29%20y%20coordinate%20%5Cu22640.07.%20The%20emitting%20excited%20state%20is%20characterized%20by%20a%20few%20nanoseconds%20lifetime%20and%20high%20radiative%20rate%20constant%2C%20and%20its%20nature%20is%20modulated%20from%20pure%20pi-pi%2A%20to%20intramolecular%20charge%20transfer%20%28ICT%29%20by%20the%20electronic%20properties%20of%20the%20peripheral%20X%20substituent.%20This%20is%20further%20corroborated%20by%20the%20nature%20of%20the%20frontier%20orbitals%20and%20vertical%20electronic%20excitations%20computed%20at%20%28time-dependent%29%20density%20functional%20level%20of%20theory%20%28TD-%29DFT.%20Finally%2C%20this%20study%20enlarges%20the%20palette%20of%20bright%20deep-blue%20emitters%20based%20on%20the%20interesting%20ImPy%20scaffolds%20in%20view%20of%20their%20potential%20application%20as%20photo-functional%20materials%20in%20optoelectronics.%22%2C%22date%22%3A%222022%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1002%5C%2Fasia.202200903%22%2C%22ISSN%22%3A%221861-471X%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1002%5C%2Fasia.202200903%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222022-11-21T10%3A05%3A34Z%22%7D%7D%2C%7B%22key%22%3A%227VN4HTLH%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Jouaiti%20et%20al.%22%2C%22parsedDate%22%3A%222022%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EA.%20Jouaiti%2C%20V.%20Giuso%2C%20C.%20Cebri%2C%20P.%20Mercandelli%2C%20M.%20Mauro%2C%20Linear%2C%20two-%20and%20four-armed%20pyridine-decorated%20thiazolo%5B5%2C4-d%5Dthiazole%20fluorophores%3A%20Synthesis%2C%20photophysical%20study%20and%20computational%20investigation%2C%20Dyes%20and%20Pigments%20208%20%282022%29%20110780.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.dyepig.2022.110780%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.dyepig.2022.110780%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Linear%2C%20two-%20and%20four-armed%20pyridine-decorated%20thiazolo%5B5%2C4-d%5Dthiazole%20fluorophores%3A%20Synthesis%2C%20photophysical%20study%20and%20computational%20investigation%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Abdelaziz%22%2C%22lastName%22%3A%22Jouaiti%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Valerio%22%2C%22lastName%22%3A%22Giuso%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Cristina%22%2C%22lastName%22%3A%22Cebri%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Pierluigi%22%2C%22lastName%22%3A%22Mercandelli%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matteo%22%2C%22lastName%22%3A%22Mauro%22%7D%5D%2C%22abstractNote%22%3A%22A%20novel%20family%20of%20thiazolo%5B5%2C4-d%5Dthiazole%20%28TzTz%29%20fluorophores%20having%20a%20general%20formula%20%28py%29n-rc-TzTz-rc-%28py%29n%20%28n%20%3D%201%20or%202%2C%20py%20%3D%20pyridine%29%20is%20herein%20reported.%20The%20investigated%20compounds%20are%20decorated%20with%20alkoxy-substituted%20pyridine%20scaffolds%20bearing%20chains%20with%20different%20lengths%20%28a%20%3D%20methoxy%2C%20b%20%3D%202-ethylhexan-1-oxy%2C%20c%20%3D%202-decyltetradecan-1-oxy%29%20that%20are%20connected%20at%20either%20the%20para-%20or%20meta-position%28s%29%20of%20the%20phenyl%20bridging%20ring.%20The%20compounds%20feature%20either%20a%20linear%20%28n%20%3D%201%2C%20para-substituted%2C%20series%208%29%2C%20two-armed%20%28n%20%3D%201%2C%20meta-substituted%20series%209%29%20or%20four-armed%20%28n%20%3D%202%2C%20meta-substituted%20series%2010%29%20molecular%20architecture.%20Steady-state%20and%20time-resolved%20photophysical%20investigation%20is%20employed%20to%20characterize%20the%20optical%20properties%20of%20the%20compounds%20in%20both%20diluted%20CH2Cl2%20and%20CH2Cl2%3Atrifluoroacetic%20acid%20%28TFA%29%2095%3A5%20solutions%2C%20as%20well%20as%20in%20thin-film%20in%20poly%28methyl%20methacrylate%29%20%28PMMA%29%20matrix%20at%2020%20wt%25%20doping.%20All%20compounds%20display%20electronic%20transitions%20with%20mainly%20rc-rc%2A%20nature%20at%20both%20solution%20and%20solid%20state.%20However%2C%20while%20both%20peripheral%20pyridyl-phenyl%20moieties%20and%20the%20rc-TzTz-rc%20core%20participate%20in%20the%20absorption%20transitions%2C%20the%20emission%20involves%20mostly%20the%20rc-TzTz-rc%20core.%20A%20clear%20effect%20of%20the%20positional%20isomerism%20on%20the%20photophysical%20properties%20is%20observed%2C%20with%20the%20protonation%20of%20the%20peripheral%20pyridines%20offering%20a%20further%20modulation.%20Thus%2C%20these%20results%20provide%20some%20useful%20hints%20on%20the%20molecular%20design%20of%20TzTz-based%20compounds%20as%20promising%20photoactive%20materials.%22%2C%22date%22%3A%222022%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.dyepig.2022.110780%22%2C%22ISSN%22%3A%220143-7208%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1016%5C%2Fj.dyepig.2022.110780%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222022-11-21T10%3A04%3A52Z%22%7D%7D%2C%7B%22key%22%3A%22YANS4X9Z%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22McCartin%20et%20al.%22%2C%22parsedDate%22%3A%222022%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EC.%20McCartin%2C%20E.%20Mathieu%2C%20M.%20Dontenwill%2C%20C.%20Herold-Mende%2C%20A.%20Idbaih%2C%20A.%20Bonfiglio%2C%20M.%20Mauro%2C%20S.%20Fournel%2C%20A.%20Kichler%2C%20An%20N-heterocyclic%20carbene%20iridium%28III%29%20complex%20as%20a%20potent%20anti-cancer%20stem%20cell%20therapeutic.%2C%20Chemico-Biological%20Interactions%20367%20%282022%29%20110167.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.cbi.2022.110167%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.cbi.2022.110167%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22An%20N-heterocyclic%20carbene%20iridium%28III%29%20complex%20as%20a%20potent%20anti-cancer%20stem%20cell%20therapeutic.%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Conor%22%2C%22lastName%22%3A%22McCartin%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Eric%22%2C%22lastName%22%3A%22Mathieu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Monique%22%2C%22lastName%22%3A%22Dontenwill%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Christel%22%2C%22lastName%22%3A%22Herold-Mende%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ahmed%22%2C%22lastName%22%3A%22Idbaih%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Anna%22%2C%22lastName%22%3A%22Bonfiglio%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matteo%22%2C%22lastName%22%3A%22Mauro%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sylvie%22%2C%22lastName%22%3A%22Fournel%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Kichler%22%7D%5D%2C%22abstractNote%22%3A%22Cancer%20stem%20cells%20%28CSCs%29%20represent%20a%20difficult%20to%20treat%20cellular%20niche%20within%20tumours%20due%20to%20their%20unique%20characteristics%2C%20which%20give%20them%20a%20high%20propensity%20for%20resistance%20to%20classical%20anti-cancer%20treatments%20and%20the%20ability%20to%20repopulate%20the%20tumour%20mass.%20An%20attribute%20that%20may%20be%20implicated%20in%20the%20high%20rates%20of%20recurrence%20of%20certain%20tumours.%20However%2C%20other%20characteristics%20specific%20to%20these%20cells%2C%20such%20as%20their%20high%20dependence%20on%20mitochondria%2C%20may%20be%20exploited%20for%20the%20development%20of%20new%20therapeutic%20agents%20that%20are%20effective%20against%20the%20niche.%20As%20such%2C%20a%20previously%20described%20phosphorescent%20N-heterocyclic%20carbene%20iridium%28III%29%20compound%20which%20showed%20a%20high%20level%20of%20cytotoxicity%20against%20classical%20tumour%20cell%20lines%20with%20mitochondria-specific%20effects%20was%20studied%20for%20its%20potential%20against%20CSCs.%20The%20results%20showed%20a%20significantly%20higher%20level%20of%20activity%20against%20several%20CSC%20lines%20compared%20to%20non-CSCs.%20Mitochondrial%20localisation%20and%20superoxide%20production%20were%20confirmed.%20Although%20the%20cell%20death%20involved%20caspase%20activation%2C%20their%20role%20in%20cell%20death%20was%20not%20definitive%2C%20with%20a%20potential%20implication%20of%20other%2C%20non-apoptotic%20pathways%20shown.%20A%20cytostatic%20effect%20of%20the%20compound%20was%20also%20displayed%20at%20low%20mortality%20doses.%20This%20study%20thus%20provides%20important%20insights%20into%20the%20mechanisms%20and%20the%20potential%20for%20this%20class%20of%20molecule%20in%20the%20domain%20of%20anti-CSC%20therapeutics.%22%2C%22date%22%3A%222022%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.cbi.2022.110167%22%2C%22ISSN%22%3A%221872-7786%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1016%5C%2Fj.cbi.2022.110167%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222022-11-16T15%3A12%3A20Z%22%7D%7D%2C%7B%22key%22%3A%22PIW6NTE6%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22McCartin%20et%20al.%22%2C%22parsedDate%22%3A%222022%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EC.%20McCartin%2C%20C.%20Dussouillez%2C%20C.%20Bernhard%2C%20E.%20Mathieu%2C%20J.%20Blumberger%2C%20M.%20Dontenwill%2C%20C.%20Herold-Mende%2C%20A.%20Idbaih%2C%20P.%20Lavalle%2C%20S.%20Bellemin-Laponnaz%2C%20A.%20Kichler%2C%20S.%20Fournel%2C%20Polyethylenimine%2C%20an%20Autophagy-Inducing%20Platinum-Carbene-Based%20Drug%20Carrier%20with%20Potent%20Toxicity%20towards%20Glioblastoma%20Cancer%20Stem%20Cells.%2C%20Cancers%2014%20%282022%29%205057.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fcancers14205057%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fcancers14205057%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Polyethylenimine%2C%20an%20Autophagy-Inducing%20Platinum-Carbene-Based%20Drug%20Carrier%20with%20Potent%20Toxicity%20towards%20Glioblastoma%20Cancer%20Stem%20Cells.%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Conor%22%2C%22lastName%22%3A%22McCartin%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Candice%22%2C%22lastName%22%3A%22Dussouillez%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Chloe%22%2C%22lastName%22%3A%22Bernhard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Eric%22%2C%22lastName%22%3A%22Mathieu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Juliette%22%2C%22lastName%22%3A%22Blumberger%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Monique%22%2C%22lastName%22%3A%22Dontenwill%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Christel%22%2C%22lastName%22%3A%22Herold-Mende%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ahmed%22%2C%22lastName%22%3A%22Idbaih%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Philippe%22%2C%22lastName%22%3A%22Lavalle%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Kichler%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sylvie%22%2C%22lastName%22%3A%22Fournel%22%7D%5D%2C%22abstractNote%22%3A%22The%20difficulty%20involved%20in%20the%20treatment%20of%20many%20tumours%20due%20to%20their%20recurrence%20and%20resistance%20to%20chemotherapy%20is%20tightly%20linked%20to%20the%20presence%20of%20cancer%20stem%20cells%20%28CSCs%29.%20This%20CSC%20sub-population%20is%20distinct%20from%20the%20majority%20of%20cancer%20cells%20of%20the%20tumour%20bulk.%20Indeed%2C%20CSCs%20have%20increased%20mitochondrial%20mass%20that%20has%20been%20linked%20to%20increased%20sensitivity%20to%20mitochondrial%20targeting%20compounds.%20Thus%2C%20a%20platinum-based%20polyethylenimine%20%28PEI%29%20polymer-drug%20conjugate%20%28PDC%29%20was%20assessed%20as%20a%20potential%20anti-CSC%20therapeutic%20since%20it%20has%20previously%20displayed%20mitochondrial%20accumulation.%20Our%20results%20show%20that%20CSCs%20have%20increased%20specific%20sensitivity%20to%20the%20PEI%20carrier%20and%20to%20the%20PDC.%20The%20mechanism%20of%20cell%20death%20seems%20to%20be%20necrotic%20in%20nature%2C%20with%20an%20absence%20of%20apoptotic%20markers.%20Cell%20death%20is%20accompanied%20by%20the%20induction%20of%20a%20protective%20autophagy.%20The%20interference%20in%20the%20balance%20of%20this%20pathway%2C%20which%20is%20highly%20important%20for%20CSCs%2C%20may%20be%20responsible%20for%20a%20partial%20reversion%20of%20the%20stem-like%20phenotype%20observed%20with%20prolonged%20PEI%20and%20PDC%20treatment.%20Several%20markers%20also%20indicate%20the%20cell%20death%20mode%20to%20be%20capable%20of%20inducing%20an%20anti-cancer%20immune%20response.%20This%20study%20thus%20indicates%20the%20potential%20therapeutic%20perspectives%20of%20polycations%20against%20CSCs.%22%2C%22date%22%3A%222022%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.3390%5C%2Fcancers14205057%22%2C%22ISSN%22%3A%222072-6694%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.3390%5C%2Fcancers14205057%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222022-11-21T10%3A12%3A31Z%22%7D%7D%2C%7B%22key%22%3A%22WEPYIDAY%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Paolino%20et%20al.%22%2C%22parsedDate%22%3A%222022%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EM.%20Paolino%2C%20M.%20Saletti%2C%20A.%20Reale%2C%20V.%20Razzano%2C%20G.%20Giuliani%2C%20A.%20Donati%2C%20C.%20Bonechi%2C%20G.%20Giorgi%2C%20A.%20Atrei%2C%20M.%20Mauro%2C%20A.%20Scamporrino%2C%20F.%20Samperi%2C%20E.%20Fois%2C%20G.%20Tabacchi%2C%20C.%20Botta%2C%20A.%20Cappelli%2C%20Spontaneous%20polymerization%20of%20benzofulvene%20derivatives%20bearing%20complexed%20or%20un-complexed%20pyridine%20rings%2C%20European%20Polymer%20Journal%20169%20%282022%29%20111137.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.eurpolymj.2022.111137%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.eurpolymj.2022.111137%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Spontaneous%20polymerization%20of%20benzofulvene%20derivatives%20bearing%20complexed%20or%20un-complexed%20pyridine%20rings%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Marco%22%2C%22lastName%22%3A%22Paolino%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Mario%22%2C%22lastName%22%3A%22Saletti%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Annalisa%22%2C%22lastName%22%3A%22Reale%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Vincenzo%22%2C%22lastName%22%3A%22Razzano%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Germano%22%2C%22lastName%22%3A%22Giuliani%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Alessandro%22%2C%22lastName%22%3A%22Donati%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Claudia%22%2C%22lastName%22%3A%22Bonechi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Gianluca%22%2C%22lastName%22%3A%22Giorgi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Andrea%22%2C%22lastName%22%3A%22Atrei%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matteo%22%2C%22lastName%22%3A%22Mauro%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Andrea%22%2C%22lastName%22%3A%22Scamporrino%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Filippo%22%2C%22lastName%22%3A%22Samperi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ettore%22%2C%22lastName%22%3A%22Fois%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Gloria%22%2C%22lastName%22%3A%22Tabacchi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Chiara%22%2C%22lastName%22%3A%22Botta%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Andrea%22%2C%22lastName%22%3A%22Cappelli%22%7D%5D%2C%22abstractNote%22%3A%22Benzofulvene%20derivatives%20bearing%20complexed%20and%20un-complexed%20pyridine%20rings%20are%20designed%20and%20synthesized%20to%20assess%20the%20effects%20on%20the%20spontaneous%20solid-state%20polymerization%20of%20the%20presence%20in%20position%206%20of%20the%203-phenylbenzofulvene%20moiety%20of%20bulky%20substituents%20capable%20of%20establishing%20metallophilic%20interactions.%20Both%20the%20benzofulvene%20monomers%20are%20found%20to%20polymerize%20spontaneously%20upon%20solvent%20removal%20under%20reduced%20pressure%20in%20the%20apparent%20absence%20of%20catalysts%20or%20initiators.%20The%20resulting%20polybenzofulvene%20derivatives%20are%20characterized%20by%20NMR%20spectroscopy%2C%20MALDI-TOF%20mass%20spectrometry%2C%20and%20in%20photophysical%20studies.%22%2C%22date%22%3A%222022%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.eurpolymj.2022.111137%22%2C%22ISSN%22%3A%220014-3057%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1016%5C%2Fj.eurpolymj.2022.111137%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222022-06-02T12%3A20%3A47Z%22%7D%7D%2C%7B%22key%22%3A%22P3V63EZ4%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Thierry%20et%20al.%22%2C%22parsedDate%22%3A%222022%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ET.%20Thierry%2C%20Y.%20Geiger%2C%20S.%20Bellemin-Laponnaz%2C%20Observation%20of%20Hyperpositive%20Non-Linear%20Effect%20in%20Asymmetric%20Organozinc%20Alkylation%20in%20Presence%20of%20N-Pyrrolidinyl%20Norephedrine%2C%20Molecules%2027%20%282022%29%203780.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fmolecules27123780%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fmolecules27123780%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Observation%20of%20Hyperpositive%20Non-Linear%20Effect%20in%20Asymmetric%20Organozinc%20Alkylation%20in%20Presence%20of%20N-Pyrrolidinyl%20Norephedrine%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thibault%22%2C%22lastName%22%3A%22Thierry%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yannick%22%2C%22lastName%22%3A%22Geiger%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%5D%2C%22abstractNote%22%3A%22Phenomena%20related%20to%20asymmetric%20amplification%20are%20considered%20to%20be%20key%20to%20understanding%20the%20emergence%20of%20homochirality%20in%20life.%20In%20asymmetric%20catalysis%2C%20theoretical%20and%20experimental%20models%20have%20been%20studied%20to%20understand%20such%20chiral%20amplification%2C%20in%20particular%20based%20on%20non-linear%20effects.%20Three%20decades%20after%20the%20theoretical%20demonstration%20that%20a%20chiral%20catalyst%2C%20when%20not%20enantiopure%2C%20could%20be%20more%20enantioselective%20than%20its%20enantiopure%20counterpart%2C%20we%20show%20here%20a%20new%20experimental%20example%20of%20nonlinear%20hyperpositive%20effect.%20We%20report%20here%20our%20investigations%20in%20the%20enantioselective%20zinc-catalyzed%20alkylation%20of%20benzaldehyde%20with%20N-pyrrolidinyl%20norephedrine%20as%20partially%20resolved%20chiral%20ligand%2C%20which%20shows%20a%20significant%20hyperpositive%20non-linear%20effect.%20A%20study%20of%20the%20underlying%20mechanism%20was%20conducted%2C%20which%20allows%20us%20to%20confirm%20a%20mechanism%20that%20implies%20a%20monomeric%20and%20a%20dimeric%20complex%20both%20catalyzing%20the%20reaction%20at%20a%20steady%20state%20and%20giving%20different%20enantioselectivities.%22%2C%22date%22%3A%222022%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.3390%5C%2Fmolecules27123780%22%2C%22ISSN%22%3A%22%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.3390%5C%2Fmolecules27123780%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222022-07-21T08%3A59%3A59Z%22%7D%7D%5D%7D
[1]
D. Bissessar, J. Egly, T. Achard, P. Steffanut, M. Mauro, S. Bellemin-Laponnaz, A Stable and Photoreactive Copper-Iodide Cubane Suitable for Direct Post-Functionalization, European Journal of Inorganic Chemistry (2022) e202200101.
https://doi.org/10.1002/ejic.202200101 .
[1]
A. Bonfiglio, P.-W. Hsiao, Y. Chen, C. Gourlaouen, Q. Marchand, V. Cesar, S. Bellemin-Laponnaz, Y.-X. Wang, C.-W. Lu, C. Daniel, F. Polo, H.-C. Su, M. Mauro, Highly Emissive Red Heterobimetallic Ir-III/M-I (M-I = Cu-I and Au-I) Complexes for Efficient Light-Emitting Electrochemical Cells, Chemistry of Materials 34 (2022) 1756–1769.
https://doi.org/10.1021/acs.chemmater.1c03972 .
[1]
J. Egly, W. Chen, A. Maisse-Francois, S. Bellemin-Laponnaz, T. Achard, Half-Sandwich Ruthenium Complexes Bearing Hemilabile kappa(2)-(C,S)-Thioether-Functionalized NHC Ligands: Application to Amide Synthesis from Alcohol and Amine, European Journal of Inorganic Chemistry (2022) e202101033.
https://doi.org/10.1002/ejic.202101033 .
[1]
E. Jouaiti, V. Giuso, D. Cianfarani, N. Kyritsakas, C. Gourlaouen, M. Mauro, Bright V-Shaped bis-Imidazo[1,2-a]pyridine Fluorophores with Near-UV to Deep-Blue Emission., Chemistry, an Asian Journal (2022) e202200903–e202200903.
https://doi.org/10.1002/asia.202200903 .
[1]
A. Jouaiti, V. Giuso, C. Cebri, P. Mercandelli, M. Mauro, Linear, two- and four-armed pyridine-decorated thiazolo[5,4-d]thiazole fluorophores: Synthesis, photophysical study and computational investigation, Dyes and Pigments 208 (2022) 110780.
https://doi.org/10.1016/j.dyepig.2022.110780 .
[1]
C. McCartin, E. Mathieu, M. Dontenwill, C. Herold-Mende, A. Idbaih, A. Bonfiglio, M. Mauro, S. Fournel, A. Kichler, An N-heterocyclic carbene iridium(III) complex as a potent anti-cancer stem cell therapeutic., Chemico-Biological Interactions 367 (2022) 110167.
https://doi.org/10.1016/j.cbi.2022.110167 .
[1]
C. McCartin, C. Dussouillez, C. Bernhard, E. Mathieu, J. Blumberger, M. Dontenwill, C. Herold-Mende, A. Idbaih, P. Lavalle, S. Bellemin-Laponnaz, A. Kichler, S. Fournel, Polyethylenimine, an Autophagy-Inducing Platinum-Carbene-Based Drug Carrier with Potent Toxicity towards Glioblastoma Cancer Stem Cells., Cancers 14 (2022) 5057.
https://doi.org/10.3390/cancers14205057 .
[1]
M. Paolino, M. Saletti, A. Reale, V. Razzano, G. Giuliani, A. Donati, C. Bonechi, G. Giorgi, A. Atrei, M. Mauro, A. Scamporrino, F. Samperi, E. Fois, G. Tabacchi, C. Botta, A. Cappelli, Spontaneous polymerization of benzofulvene derivatives bearing complexed or un-complexed pyridine rings, European Polymer Journal 169 (2022) 111137.
https://doi.org/10.1016/j.eurpolymj.2022.111137 .
[1]
T. Thierry, Y. Geiger, S. Bellemin-Laponnaz, Observation of Hyperpositive Non-Linear Effect in Asymmetric Organozinc Alkylation in Presence of N-Pyrrolidinyl Norephedrine, Molecules 27 (2022) 3780.
https://doi.org/10.3390/molecules27123780 .
1839302
ITCCYZMF
2021
1
surface-science-reports
50
creator
asc
year
10207
https://www.ipcms.fr/plugins/zotpress/
%7B%22status%22%3A%22success%22%2C%22updateneeded%22%3Afalse%2C%22instance%22%3Afalse%2C%22meta%22%3A%7B%22request_last%22%3A0%2C%22request_next%22%3A0%2C%22used_cache%22%3Atrue%7D%2C%22data%22%3A%5B%7B%22key%22%3A%22HYZRC4U7%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Achard%20and%20Bellemin-Laponnaz%22%2C%22parsedDate%22%3A%222021%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ET.%20Achard%2C%20S.%20Bellemin-Laponnaz%2C%20Recent%20Advances%20on%20Catalytic%20Osmium-Free%20Olefin%20syn-Dihydroxylation%2C%20European%20Journal%20of%20Organic%20Chemistry%202021%20%282021%29%20877%26%23x2013%3B896.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fejoc.202001209%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fejoc.202001209%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Recent%20Advances%20on%20Catalytic%20Osmium-Free%20Olefin%20syn-Dihydroxylation%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thierry%22%2C%22lastName%22%3A%22Achard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%5D%2C%22abstractNote%22%3A%22The%20syn-dihydroxylation%20of%20olefinic%20group%20is%20one%20of%20the%20most%20important%20synthetic%20transformations%20which%20leads%20to%20the%20controlled%20formation%20of%20syn-diol.%20Such%201%2C2-diol%20structure%20is%20integrated%20into%20more%20complex%20architectures%20and%20substantially%20participates%20in%20the%20development%20of%20biologically%20active%20molecules%20and%20other%20fine%20chemicals.%20This%20minireview%20describes%20recent%20evolution%20of%20this%20research%20field%20in%20the%20past%20decade%2C%20surveying%20osmium-free%20and%20more%20eco-compatible%20system%20and%20in%20particular%20the%20direct%20oxidation%20of%20olefins%20catalyzed%20by%20transition%20metal%20complexes.%22%2C%22date%22%3A%222021%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1002%5C%2Fejoc.202001209%22%2C%22ISSN%22%3A%221434-193X%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1002%5C%2Fejoc.202001209%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22BMA9GKQT%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222022-01-20T08%3A42%3A34Z%22%7D%7D%2C%7B%22key%22%3A%22DB9RGV7K%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Asbai%20et%20al.%22%2C%22parsedDate%22%3A%222021%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EZ.%20Asbai%2C%20A.%20Bonfiglio%2C%20P.%20Mercandelli%2C%20F.%20Polo%2C%20M.%20Mauro%2C%20Cationic%20rhenium%28I%29%20complexes%20bearing%20a%20pi-accepting%20pyridoannulated%20N-heterocyclic%20carbene%20ligand%3A%20Synthesis%2C%20photophysical%2C%20electrochemical%20and%20theoretical%20investigation%2C%20Polyhedron%20197%20%282021%29%20115025.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.poly.2021.115025%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.poly.2021.115025%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Cationic%20rhenium%28I%29%20complexes%20bearing%20a%20pi-accepting%20pyridoannulated%20N-heterocyclic%20carbene%20ligand%3A%20Synthesis%2C%20photophysical%2C%20electrochemical%20and%20theoretical%20investigation%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Zakariae%22%2C%22lastName%22%3A%22Asbai%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Anna%22%2C%22lastName%22%3A%22Bonfiglio%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Pierluigi%22%2C%22lastName%22%3A%22Mercandelli%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Federico%22%2C%22lastName%22%3A%22Polo%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matteo%22%2C%22lastName%22%3A%22Mauro%22%7D%5D%2C%22abstractNote%22%3A%22A%20novel%20family%20of%20photoactive%20cationic%20tris-carbonyl%20rhenium%20complexes%20of%20general%20formula%20fac-%5BRe%20%28CO%29%283%29%28PYIPY%29%28L%29%5DPF6%2C%20where%20pyipy%20is%20the%202-%28pyridyl%29-imidazo%5B1%2C5-a%5Dpyridyl-3-ylidene%20ligand%20and%20L%20%3D%20pyridine%20%283.PF6%29%20or%20PPh3%20%284.PF6%29%2C%20is%20herein%20presented.%20Compounds%203.PF6%20and%204.PF6%20are%20straightfor-wardly%20synthetized%20from%20the%20corresponding%20chloro-derivativefac-%5BReCl%28CO%29%283%29%20%28pyipy%29%5D%20via%20halogen%20abstraction%20with%20silver%28l%29%20salt%2C%20followed%20by%20coordination%20of%20the%20neutral%20monodentate%20L%20ligand.%20The%20target%20complexes%20were%20characterized%20by%20H-1%20and%20C-13%20NMR%20as%20well%20as%20FT-IR%20spectroscopy%20and%20high-resolution%20mass%20spectrometry%20%28HR-MS%29.%20In%20addition%2C%20X-ray%20diffractometric%20analysis%20was%20carried%20out%20by%20solving%20the%20single-crystal%20structure%20of%20compound%203.PF6.%20For%20both%20complexes%2C%20dilute%20samples%20in%20CH3CN%20solution%20at%20room%20temperature%20display%20a%20structured%20photoluminescence%20profile%20in%20the%20red%20region%20that%20is%20ascribed%20to%20a%20long-lived%20excited%20state%20tau%20%3D%2019.3-30.0%20mu%20s%20in%20degassed%20condition%29%20with%20mainly%20triplet%20ligand-centered%20%28%28LC%29-L-3%29%20character.%20This%20assignment%20is%20further%20corroborated%20by%20the%20minor%20hypsochromic%20shift%20of%20the%20emission%20profile%20observed%20for%20samples%20in%202-MeTHF%20at%2077%20K%20glassy%20matrix%2C%20in%20agreement%20with%20previous%20studies%20on%20the%20corresponding%20neutral%20counterparts.%20Electrochemical%20characterization%20by%20cyclic%20voltammetry%20%28CV%29%20shows%20two%20irreversible%20processes%2C%20one%20in%20the%20positive%20and%20one%20in%20the%20negative%20bias%2C%20which%20are%20associated%20to%20the%20metal-NHC%20moiety%20and%20the%20chelating%20NHC%20ligand%2C%20respectively.%20Finally%2C%20both%20electronic%20and%20optical%20features%20are%20further%20studied%20by%20means%20of%20computational%20investigation%20at%20density%20functional%20theory%20%28DFT%29%20and%20time-dependent%20DFT%20%28TD-DFT%29%20level%20of%20theory%2C%20which%20supports%20the%20attribution%20of%20the%20electronic%20transitions%20involved.%20%28C%29%202021%20Elsevier%20Ltd.%20All%20rights%20reserved.%22%2C%22date%22%3A%222021%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.poly.2021.115025%22%2C%22ISSN%22%3A%220277-5387%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1016%5C%2Fj.poly.2021.115025%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222021-05-11T08%3A56%3A19Z%22%7D%7D%2C%7B%22key%22%3A%2227GQBFWX%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Bonfiglio%20et%20al.%22%2C%22parsedDate%22%3A%222021%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EA.%20Bonfiglio%2C%20C.%20McCartin%2C%20U.%20Carrillo%2C%20C.%20Cebrian%2C%20P.C.%20Gros%2C%20S.%20Fournel%2C%20A.%20Kichler%2C%20C.%20Daniel%2C%20M.%20Mauro%2C%20Ir-III-Pyridoannelated%20N-Heterocyclic%20Carbene%20Complexes%3A%20Potent%20Theranostic%20Agents%20via%20Mitochondria%20Targeting%2C%20European%20Journal%20of%20Inorganic%20Chemistry%20%282021%29%201551%26%23x2013%3B1564.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fejic.202100132%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fejic.202100132%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Ir-III-Pyridoannelated%20N-Heterocyclic%20Carbene%20Complexes%3A%20Potent%20Theranostic%20Agents%20via%20Mitochondria%20Targeting%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Anna%22%2C%22lastName%22%3A%22Bonfiglio%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Conor%22%2C%22lastName%22%3A%22McCartin%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ulises%22%2C%22lastName%22%3A%22Carrillo%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Cristina%22%2C%22lastName%22%3A%22Cebrian%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Philippe%20C.%22%2C%22lastName%22%3A%22Gros%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sylvie%22%2C%22lastName%22%3A%22Fournel%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Kichler%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Chantal%22%2C%22lastName%22%3A%22Daniel%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matteo%22%2C%22lastName%22%3A%22Mauro%22%7D%5D%2C%22abstractNote%22%3A%22A%20novel%20family%20of%20Ir-III%20complexes%20bearing%20a%20pyridoannelated%20N-heterocyclic%20carbene%20is%20herein%20described.%20The%20target%20compounds%201-5%20are%20straightforwardly%20prepared%20and%20fully%20characterized.%20Comprehensive%20investigation%20of%20their%20optical%20properties%20reveals%20a%20rare%20case%20of%20dual%20emission%20ascribed%20to%20two%20excited%20states%20localized%20onto%20different%20portions%20of%20the%20molecule%2C%20as%20confirmed%20by%20both%20optical%20spectroscopy%20and%20time-dependent%20density%20functional%20calculations%20including%20spin-orbit%20coupling.%20The%20cytotoxicity%20against%20cancer%20cell%20lines%20is%20investigated%20as%20well.%20Remarkably%2C%202-5%20show%20up%20to%2050-fold%20higher%20anticancer%20activity%20in%20vitro%20compared%20to%20oxaliplatin%20market%20drug%20with%20concentration%20values%20required%20to%20reduce%20by%2050%20%25%20the%20cell%20viability%20%28IC50%29%20in%20the%20low%20mu%20M%20range.%20Finally%2C%20in-depth%20biological%20investigation%20on%20the%20most%20cytotoxic%20compound%204%20reveals%20its%20powerful%20mitochondrial%20dysfunctioning%20activity%20and%20efficient%20reactive%20oxygen%20species%20production%2C%20associated%20with%20apoptosis%20as%20the%20mechanism%20of%20cell%20death.%22%2C%22date%22%3A%222021%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1002%5C%2Fejic.202100132%22%2C%22ISSN%22%3A%221434-1948%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1002%5C%2Fejic.202100132%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222025-05-27T11%3A38%3A02Z%22%7D%7D%2C%7B%22key%22%3A%22WUDRAHXQ%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Chen%20et%20al.%22%2C%22parsedDate%22%3A%222021%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EH.%20Chen%2C%20Y.%20Zhang%2C%20A.%20Bonfiglio%2C%20F.%20Morlet-Savary%2C%20M.%20Mauro%2C%20J.%20Lalevee%2C%20Rhenium%28I%29%20N-Heterocyclic%20Carbene%20Complexes%20in%20Photoinitiating%20Systems%20for%20Polymerization%20upon%20Visible%20Light%3A%20Development%20of%20Photosensitive%20Resins%20for%203D%20and%204D%20Applications%2C%20ACS%20Applied%20Polymer%20Materials%203%20%282021%29%20464%26%23x2013%3B473.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1021%5C%2Facsapm.0c01207%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1021%5C%2Facsapm.0c01207%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Rhenium%28I%29%20N-Heterocyclic%20Carbene%20Complexes%20in%20Photoinitiating%20Systems%20for%20Polymerization%20upon%20Visible%20Light%3A%20Development%20of%20Photosensitive%20Resins%20for%203D%20and%204D%20Applications%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Hong%22%2C%22lastName%22%3A%22Chen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yijun%22%2C%22lastName%22%3A%22Zhang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Anna%22%2C%22lastName%22%3A%22Bonfiglio%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Fabrice%22%2C%22lastName%22%3A%22Morlet-Savary%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matteo%22%2C%22lastName%22%3A%22Mauro%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jacques%22%2C%22lastName%22%3A%22Lalevee%22%7D%5D%2C%22abstractNote%22%3A%22Two%20fac-rhenium%20tris-carbonyl%20complexes%20bearing%20a%20pi-accepting%20pyridoannelated%20N-heterocyclic%20carbene%20%28NHC%29%20ligand%20with%20general%20formula%20Re%28CO%29%283%29%20%28pyipy%29X%2C%20where%20pyipy%20is%202-pyridil-imidazo%5B1%2C5-a%5Dpyridine-3-ylidene%20and%20X%20%3D%20CI%20%28Re1%29%20and%20Br%20%28Re2%29%2C%20are%20investigated%20as%20photoredox%20catalytic%20systems%20in%20light-promoted%20polymerization%20reactions.%20Both%20rhenium%28I%29%20complexes%20are%20able%20to%20efficiently%20generate%20radicals%20to%20initiate%20free%20radical%20polymerization.%20Remarkably%2C%20excellent%20radical%20polymerization%20profiles%20and%20final%20conversions%20are%20obtained%20under%20soft%20irradiations%20%28LED%20lamp%2C%20lambda%28exc%29%20%3D%20405%20nm%29%20by%20using%20the%20proposed%20complexes%20with%20iodonium%20salt%20and%20amine%20as%20photoinitiating%20systems%20%28PISs%29%20for%20polyethyleneglycol%20%28PEG%29-acrylate%20monomers.%20The%20involved%20mechanism%20is%20investigated%20by%20means%20of%20different%20spectroscopic%20techniques%20and%20further%20supported%20by%20electrochemical%20data.%20In%20addition%2C%20using%20the%20prepared%20three-component%20PISs%2C%20outstanding%203D%20patterns%20with%20spatial%20resolution%20could%20be%20obtained%20through%20direct%20laser%20write%20experiments.%20Remarkably%2C%20the%20resulting%20material%20displays%20reversible%20deformation%20that%20can%20be%20controlled%20with%20heating-hydration%20cycles%20due%20to%20stimuli-responsiveness%20of%20PEG%20chains%20of%20the%20polymeric%20network%2C%20thus%20opening%20the%20way%20for%20innovative%20smart%20and%20stimuli-responsive%204D-printed%20materials%20and%20soft%20actuators.%22%2C%22date%22%3A%222021%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1021%5C%2Facsapm.0c01207%22%2C%22ISSN%22%3A%222637-6105%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1021%5C%2Facsapm.0c01207%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222021-08-17T07%3A18%3A35Z%22%7D%7D%2C%7B%22key%22%3A%22SDJVXC9J%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22De%20Marco%20et%20al.%22%2C%22parsedDate%22%3A%222021%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ER.%20De%20Marco%2C%20M.%20Dal%20Grande%2C%20M.%20Baron%2C%20L.%20Orian%2C%20C.%20Graiff%2C%20T.%20Achard%2C%20S.%20Bellemin-Laponnaz%2C%20A.%20Pothig%2C%20C.%20Tubaro%2C%20Synthesis%2C%20Structural%20Characterization%20and%20Antiproliferative%20Activity%20of%20Gold%28I%29%20and%20Gold%28III%29%20Complexes%20Bearing%20Thioether-Functionalized%20N-Heterocyclic%20Carbenes%2C%20European%20Journal%20of%20Inorganic%20Chemistry%202021%20%282021%29%204196%26%23x2013%3B4206.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fejic.202100495%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fejic.202100495%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Synthesis%2C%20Structural%20Characterization%20and%20Antiproliferative%20Activity%20of%20Gold%28I%29%20and%20Gold%28III%29%20Complexes%20Bearing%20Thioether-Functionalized%20N-Heterocyclic%20Carbenes%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Riccardo%22%2C%22lastName%22%3A%22De%20Marco%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Marco%22%2C%22lastName%22%3A%22Dal%20Grande%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Marco%22%2C%22lastName%22%3A%22Baron%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Laura%22%2C%22lastName%22%3A%22Orian%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Claudia%22%2C%22lastName%22%3A%22Graiff%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thierry%22%2C%22lastName%22%3A%22Achard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Alexander%22%2C%22lastName%22%3A%22Pothig%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Cristina%22%2C%22lastName%22%3A%22Tubaro%22%7D%5D%2C%22abstractNote%22%3A%22A%20series%20of%20gold%28I%29%20and%20gold%28III%29%20complexes%20with%20N-heterocyclic%20carbene%20ligands%20functionalized%20with%20a%20pendant%20thioether%20group%20%28NHC-SR%29%20was%20synthesized%20with%20straightforward%20procedures%20and%20characterized%20in%20solution%20with%20NMR%20spectroscopy%20and%20ESI-MS%20spectrometry%2C%20as%20well%20as%20in%20the%20solid%20state%20by%20means%20of%20single%20crystal%20X-ray%20diffraction%20analysis.%20Selected%20experimental%20aspects%20were%20rationalized%20through%20relativistic%20DFT%20calculations.%20The%20gold%28I%29%20and%20gold%28III%29%20complexes%20displayed%20moderate%20in%20vitro%20cytotoxicity%20towards%20breast%20cancer%20cells%20MCF7.%22%2C%22date%22%3A%222021%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1002%5C%2Fejic.202100495%22%2C%22ISSN%22%3A%221434-1948%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1002%5C%2Fejic.202100495%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222025-04-15T07%3A35%3A44Z%22%7D%7D%2C%7B%22key%22%3A%226BRMPSFP%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Egly%20et%20al.%22%2C%22parsedDate%22%3A%222021%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EJ.%20Egly%2C%20D.%20Bissessar%2C%20T.%20Achard%2C%20B.%20Heinrich%2C%20P.%20Steffanut%2C%20M.%20Mauro%2C%20S.%20Bellemin-Laponnaz%2C%20Copper%28I%29%20complexes%20with%20remotely%20functionalized%20phosphine%20ligands%3A%20Synthesis%2C%20structural%20variety%2C%20photophysics%20and%20effect%20onto%20the%20optical%20properties%2C%20Inorganica%20Chimica%20Acta%20514%20%282021%29%20119971.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.ica.2020.119971%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.ica.2020.119971%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Copper%28I%29%20complexes%20with%20remotely%20functionalized%20phosphine%20ligands%3A%20Synthesis%2C%20structural%20variety%2C%20photophysics%20and%20effect%20onto%20the%20optical%20properties%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Julien%22%2C%22lastName%22%3A%22Egly%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Damien%22%2C%22lastName%22%3A%22Bissessar%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thierry%22%2C%22lastName%22%3A%22Achard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Benoit%22%2C%22lastName%22%3A%22Heinrich%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Pascal%22%2C%22lastName%22%3A%22Steffanut%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matteo%22%2C%22lastName%22%3A%22Mauro%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%5D%2C%22abstractNote%22%3A%22The%20synthesis%2C%20chemical%20and%20photophysical%20investigation%20of%20a%20series%20of%20eight%20novel%20copper-halide%20derivatives%20with%20different%20nuclearity%20is%20herein%20presented.%20One%20mononuclear%20copper%28I%29%20complex%20with%20formula%20%5BCuI%28pyridine%29%28P%29%282%29%5D%2C%20where%20P%20is%20a%20functionalized%20phosphine%2C%20six%20dinuclear%20derivatives%20bearing%20the%20rhombic%20%7BCu2I2%7D%20subunits%20and%20with%20general%20formula%20%5BCu%28mu-I%29%28P%29%28N%29%282%29%20%28N%20%3D%20pyridine%2C%20quinoline%20and%204-cyanopyridine%29%20as%20well%20as%20one%20tetranuclear%20copper-iodide%20clusters%20of%20general%20formula%20%5BCu%28mu-I%29P%5D%284%29%20consisting%20of%20a%20cubane-like%20%7BCu4X4%7D%20motif%20were%20straightforwardly%20prepared%2C%20also%20by%20means%20of%20mechano-chemical%20synthetic%20procedure.%20The%20phosphine%20ligands%20were%20prepared%20in%20excellent%20yield%20by%20using%20simple%20hydrophosphination%20reactions.%20For%20five%20of%20the%20investigated%20cuprous%20iodide%20derivatives%20their%20atom%20connectivity%20and%20solid-state%20crystalline%20packing%20was%20unambiguously%20confirmed%20by%20solving%20their%20single-crystal%20X-ray%20structure.%20All%20the%20investigated%20complexes%20resulted%20into%20photo-%20and%20thermallystable%20luminescent%20species.%20In%20the%20solid%20state%20as%20microcrystalline%20powder%20samples%2C%20the%20complexes%20display%20intense%20photoluminescence%20ranging%20from%20the%20sky-blue%20to%20orange-red%20portion%20of%20the%20spectrum%20with%20PLQY%20values%20of%200.28-0.50%20and%200.34-0.97%20at%20room%20and%20low%20%2877%20K%29%20temperature%2C%20respectively%2C%20depending%20on%20the%20nature%20of%20both%20the%20coordinated%20N-heteroaromatic%20ring%20and%20phosphine%20ligands.%20In%20spite%20of%20the%20remote%20location%20of%20the%20functionalization%20of%20the%20phosphine%2C%20profound%20effects%20are%20observed%20onto%20the%20solid-state%20emission%20properties%20highlighting%20the%20importance%20of%20microenvironment%20for%20the%20emitters%20in%20the%20aggregated%20phase.%22%2C%22date%22%3A%222021%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.ica.2020.119971%22%2C%22ISSN%22%3A%220020-1693%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1016%5C%2Fj.ica.2020.119971%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22BMA9GKQT%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222021-04-30T13%3A56%3A03Z%22%7D%7D%2C%7B%22key%22%3A%22HBIABVMM%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Geiger%20et%20al.%22%2C%22parsedDate%22%3A%222021%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EY.%20Geiger%2C%20T.%20Achard%2C%20A.%20Maisse-Francois%2C%20S.%20Bellemin-Laponnaz%2C%20Absence%20of%20Non-Linear%20Effects%20Despite%20Evidence%20for%20Catalyst%20Aggregation%2C%20European%20Journal%20of%20Organic%20Chemistry%20%282021%29%202916%26%23x2013%3B2922.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fejoc.202100183%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fejoc.202100183%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Absence%20of%20Non-Linear%20Effects%20Despite%20Evidence%20for%20Catalyst%20Aggregation%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yannick%22%2C%22lastName%22%3A%22Geiger%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thierry%22%2C%22lastName%22%3A%22Achard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Aline%22%2C%22lastName%22%3A%22Maisse-Francois%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%5D%2C%22abstractNote%22%3A%22An%20in-depth%20study%20of%20the%20catalytic%20system%2C%20consisting%20of%20the%20enantioselective%20addition%20of%20ZnEt2%20to%20benzaldehyde%20with%20%281R%2C2S%29-%28-%29-N-Methylephedrine%20%28NME%29%20as%20chiral%20ligand%2C%20suggests%20the%20presence%20of%20dimeric%20and%20trimeric%20aggregates%2C%20as%20deduced%20from%20product%20ee%20vs.%20catalyst%20loading%20and%20NMR%20investigations%20%28H-1%2C%20DOSY%29.%20Formation%20of%20catalyst%20aggregation%20was%20excluded%20in%20earlier%20studies%20as%20this%20system%20displays%20a%20linear%20product%20ee%20vs.%20ligand%20ee-correlation%2C%20which%20is%20usually%20taken%20as%20an%20indication%20for%20the%20absence%20of%20catalyst%20aggregation.%20A%20subsequent%20theoretical%20study%2C%20using%20the%20monomer-dimer%20competition%20model%2C%20which%20we%20have%20recently%20developed%2C%20highlights%20the%20possible%20parameter%20configurations%20leading%20to%20linear%20product%20ee%20vs.%20ligand%20ee%20plots%20-%20despite%20the%20presence%20of%20catalyst%20dimers.%20It%20shows%20that%2C%20while%20the%20Kagan%20and%20Noyori%20models%20allow%20linearity%20in%20very%20specific%20cases%20only%2C%20a%20multitude%20of%20scenarios%20may%20lead%20to%20linearity%20here%2C%20especially%20if%20heterochiral%20dimers%20are%20catalytically%20active.%22%2C%22date%22%3A%222021%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1002%5C%2Fejoc.202100183%22%2C%22ISSN%22%3A%221434-193X%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1002%5C%2Fejoc.202100183%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222022-02-11T12%3A15%3A36Z%22%7D%7D%2C%7B%22key%22%3A%22RVZPKABZ%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Hammoud%20et%20al.%22%2C%22parsedDate%22%3A%222021%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EF.%20Hammoud%2C%20M.%20Rahal%2C%20J.%20Egly%2C%20F.%20Morlet-Savary%2C%20A.%20Hijazi%2C%20S.%20Bellemin-Laponnaz%2C%20M.%20Mauro%2C%20J.%20Lalevee%2C%20Cubane%20Cu4I4%28phosphine%29%284%29%20complexes%20as%20new%20co-initiators%20for%20free%20radical%20photopolymerization%3A%20towards%20aromatic%20amine-free%20systems%2C%20Polymer%20Chemistry%2012%20%282021%29%202848%26%23x2013%3B2859.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fd1py00277e%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fd1py00277e%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Cubane%20Cu4I4%28phosphine%29%284%29%20complexes%20as%20new%20co-initiators%20for%20free%20radical%20photopolymerization%3A%20towards%20aromatic%20amine-free%20systems%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Fatima%22%2C%22lastName%22%3A%22Hammoud%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Mahmoud%22%2C%22lastName%22%3A%22Rahal%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Julien%22%2C%22lastName%22%3A%22Egly%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Fabrice%22%2C%22lastName%22%3A%22Morlet-Savary%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Akram%22%2C%22lastName%22%3A%22Hijazi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matteo%22%2C%22lastName%22%3A%22Mauro%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jacques%22%2C%22lastName%22%3A%22Lalevee%22%7D%5D%2C%22abstractNote%22%3A%22The%20investigation%20of%20copper-iodide%20cubane%20derivatives%20as%20new%20co-initiators%20for%20the%20free%20radical%20polymerization%20%28FRP%29%20of%20acrylate%20monomers%20under%20mild%20irradiation%20conditions%20is%20described%20here%20for%20the%20first%20time.%20These%20tetranuclear%20Cu%28I%29-based%20complexes%20have%20the%20general%20formula%2C%20Cu4I4%28phosphine%29%284%29%2C%20where%20phosphine%20is%20triphenylphosphine%20%28Cu1%29%20or%20diphenylphosphine%20%28Cu2%29.%20In%20the%20presence%20of%20commercial%20Type%20II%20photoinitiators%20such%20as%20isopropylthioxanthone%20%28ITX%29%20or%20camphorquinone%20%28CQ%29%2C%20this%20class%20of%20co-initiators%20achieves%20high%20final%20conversions%20of%20the%20reactive%20functional%20groups.%20A%20comparison%20between%20the%20aromatic%20amine%2C%20namely%20ethyldimethylaminobenzoate%20%28EDB%29%2C%20used%20as%20a%20benchmark%20hydrogen%20donor%2C%20and%20these%20copper-based%20co-initiators%20is%20made%2C%20which%20demonstrates%20that%20the%20latter%20can%20be%20considered%20as%20valid%2C%20environmentally%20friendly%20alternatives%20to%20toxic%20aromatic%20amines.%20Markedly%2C%20photoluminescent%20materials%20can%20be%20generated%20thanks%20to%20the%20presence%20of%20cubanes%20linked%20to%20the%20polymer%20network.%22%2C%22date%22%3A%222021%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1039%5C%2Fd1py00277e%22%2C%22ISSN%22%3A%221759-9954%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1039%5C%2Fd1py00277e%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222021-08-17T07%3A12%3A08Z%22%7D%7D%2C%7B%22key%22%3A%22W9TAUHKU%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Le%20Roux%20et%20al.%22%2C%22parsedDate%22%3A%222021%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ES.%20Le%20Roux%2C%20G.%20Ori%2C%20S.%20Bellemin-Laponnaz%2C%20M.%20Boero%2C%20Tridentate%20complexes%20of%20group%204%20bearing%20bis-aryloxide%20N-heterocyclic%20carbene%20ligand%3A%20Structure%2C%20spin%20density%20and%20charge%20states%2C%20Chemical%20Physics%20Letters%20781%20%282021%29%20138888.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.cplett.2021.138888%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.cplett.2021.138888%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Tridentate%20complexes%20of%20group%204%20bearing%20bis-aryloxide%20N-heterocyclic%20carbene%20ligand%3A%20Structure%2C%20spin%20density%20and%20charge%20states%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sebastien%22%2C%22lastName%22%3A%22Le%20Roux%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Guido%22%2C%22lastName%22%3A%22Ori%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Mauro%22%2C%22lastName%22%3A%22Boero%22%7D%5D%2C%22abstractNote%22%3A%22Metal%20carbene%20complexes%20represent%20an%20ubiquitous%20class%20of%20compounds%20in%20organomettalic%20chemistry%20able%20to%20trigger%20a%20wealth%20of%20catalytic%20reactions%20of%20both%20fundamental%20and%20industrial%20processes.%20By%20resorting%20to%20first%20principles%20approaches%2C%20we%20focus%20on%20the%20fundamental%20features%20of%20these%20complexes%20in%20which%20the%20metal%20center%20can%20be%20either%20Hf%2C%20Ti%20or%20Zr.%20These%20specific%20systems%20show%20interesting%20features%20in%20their%20neutral%20and%20charged%20%28%2B1%29%20states.%20Yet%2C%20we%20provide%20evidence%20of%20the%20fact%20that%20the%20nature%20of%20the%20metallic%20ion%20does%20not%20have%20a%20significant%20impact%20on%20the%20structure%20of%20the%20ligand.%20Conversely%2C%20the%20removal%20of%20one%20electron%20%28charge%20state%20%2B1%29%20induces%20a%20perturbation%20of%20the%20ligand%20conformation%20and%20this%2C%20in%20turn%2C%20is%20prone%20to%20affect%20the%20catalytic%20properties%20of%20the%20complex%2C%20carrying%20the%20singly%20occupied%20electronic%20level%20%28spin%20distribution%20of%20the%20system%29%20delocalized%20over%20the%20carbene%20ligand.%22%2C%22date%22%3A%222021%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.cplett.2021.138888%22%2C%22ISSN%22%3A%220009-2614%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1016%5C%2Fj.cplett.2021.138888%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22NZSFH59F%22%2C%22TK3HH32E%22%2C%22CF4ZI7HM%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222021-10-21T09%3A49%3A44Z%22%7D%7D%2C%7B%22key%22%3A%22HLY5UAJX%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Marinova%20et%20al.%22%2C%22parsedDate%22%3A%222021%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EM.%20Marinova%2C%20A.%20Bonnefont%2C%20T.%20Achard%2C%20A.%20Maisse-Francois%2C%20S.%20Bellemin-Laponnaz%2C%20Polymerization%5C%2Fdepolymerization%20of%20chiral%20metallo-supramolecular%20assembly%20induced%20by%20redox%20change%2C%20Chirality%20%282021%29%201%26%23x2013%3B8.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fchir.23342%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fchir.23342%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Polymerization%5C%2Fdepolymerization%20of%20chiral%20metallo-supramolecular%20assembly%20induced%20by%20redox%20change%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Maya%22%2C%22lastName%22%3A%22Marinova%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Bonnefont%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thierry%22%2C%22lastName%22%3A%22Achard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Aline%22%2C%22lastName%22%3A%22Maisse-Francois%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%5D%2C%22abstractNote%22%3A%22We%20report%20on%20the%20polymerization%5C%2Fdepolymerization%20of%20chiral%20metallo-supramolecular%20assembly%20by%20Cu-I%5C%2FCu-II%20redox%20change.%20By%20combining%20a%20monotopic%20enantiopure%20ligand%20with%20a%20ditopic%20ligand%20of%20opposite%20configuration%2C%20ML2-type%20complexes%20are%20generated%20with%20chiral%20self-recognition%20or%20self-discrimination%20depending%20on%20the%20oxidation%20state%20of%20copper.%20In%20presence%20of%20Cu-I%2C%20the%20formation%20of%20heterochiral%20complexes%20is%20favored%2C%20thus%20generating%20dinuclear%20species%20whereas%20Cu-II%20advocates%20for%20the%20formation%20of%20homochiral%20species%2C%20namely%2C%20a%20mixture%20of%20mononuclear%20species%20and%20metallo-supramolecular%20polymeric%20species.%20Thus%2C%20cyclic%20voltammetry%20was%20used%20to%20study%20such%20a%20chirality-induced%20stimulus%20sensitive%20polymerization%5C%2Fdepolymerization%20process.%22%2C%22date%22%3A%222021%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1002%5C%2Fchir.23342%22%2C%22ISSN%22%3A%220899-0042%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1002%5C%2Fchir.23342%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222021-08-17T11%3A54%3A39Z%22%7D%7D%2C%7B%22key%22%3A%22I5DZWQ4N%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Mauro%22%2C%22parsedDate%22%3A%222021%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EM.%20Mauro%2C%20Phosphorescent%20multinuclear%20complexes%20for%20optoelectronics%3A%20tuning%20of%20the%20excited-state%20dynamics%2C%20Chemical%20Communications%20%282021%29%205857.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fd1cc01077h%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fd1cc01077h%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Phosphorescent%20multinuclear%20complexes%20for%20optoelectronics%3A%20tuning%20of%20the%20excited-state%20dynamics%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matteo%22%2C%22lastName%22%3A%22Mauro%22%7D%5D%2C%22abstractNote%22%3A%22Luminescent%20transition%20metal%20complexes%20have%20attracted%20a%20great%20deal%20of%20attention%20in%20the%20last%20two%20decades%20from%20both%20fundamental%20and%20application%20points%20of%20view.%20The%20majority%20of%20the%20investigated%20and%20most%20efficient%20systems%20consist%20of%20monometallic%20compounds%20with%20judiciously%20selected%20ligand%20sphere%2C%20providing%20excellent%20triplet%20emitters%20for%20both%20lab-scale%20and%20real-market%20light-emitting%20devices%20for%20display%20technologies.%20More%20recently%2C%20chemical%20architectures%20comprising%20multimetallic%20compounds%20have%20appeared%20as%20an%20emerging%20and%20valuable%20alternative.%20Herein%2C%20the%20most%20recent%20trends%20in%20the%20field%20are%20showcased%20in%20a%20systematic%20approach%2C%20where%20the%20different%20examples%20are%20classified%20by%20metal%20center%20and%20ligand%28s%29%20scaffold.%20Their%20optical%20and%20electroluminescence%20properties%20are%20presented%20and%20compared%20as%20well.%20Indeed%2C%20the%20multimetallic%20strategy%20has%20proven%20to%20be%20highly%20suitable%20for%20compounds%20emitting%20efficiently%20in%20the%20challenging%20red%20to%20near-infrared%20region%2C%20yielding%20metal-based%20emitters%20with%20improved%20optical%20properties%20in%20terms%20of%20enhanced%20emission%20efficiency%2C%20shortened%20excited-state%20lifetime%2C%20and%20faster%20radiative%20rate%20constant.%20Finally%2C%20the%20advantages%20and%20drawbacks%20of%20the%20multimetallic%20approach%20will%20be%20discussed.%22%2C%22date%22%3A%222021%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1039%5C%2Fd1cc01077h%22%2C%22ISSN%22%3A%221359-7345%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1039%5C%2Fd1cc01077h%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222022-02-11T12%3A19%3A04Z%22%7D%7D%2C%7B%22key%22%3A%22GWI6ES33%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Paolino%20et%20al.%22%2C%22parsedDate%22%3A%222021%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EM.%20Paolino%2C%20A.%20Reale%2C%20G.%20Magrini%2C%20V.%20Razzano%2C%20M.%20Saletti%2C%20G.%20Giuliani%2C%20A.%20Donati%2C%20F.%20Samperi%2C%20A.%20Scamporrino%2C%20M.%20Canetti%2C%20M.%20Mauro%2C%20F.%20Villafiorita-Monteleone%2C%20E.%20Fois%2C%20C.%20Botta%2C%20A.%20Cappelli%2C%20Synthesis%20and%20UV-light%20induced%20oligomerization%20of%20a%20benzofulvene-based%20neutral%20platinum%28II%29%20complex%2C%20European%20Polymer%20Journal%20156%20%282021%29%20110597.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.eurpolymj.2021.110597%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.eurpolymj.2021.110597%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Synthesis%20and%20UV-light%20induced%20oligomerization%20of%20a%20benzofulvene-based%20neutral%20platinum%28II%29%20complex%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Marco%22%2C%22lastName%22%3A%22Paolino%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Annalisa%22%2C%22lastName%22%3A%22Reale%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Giulia%22%2C%22lastName%22%3A%22Magrini%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Vincenzo%22%2C%22lastName%22%3A%22Razzano%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Mario%22%2C%22lastName%22%3A%22Saletti%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Germano%22%2C%22lastName%22%3A%22Giuliani%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Alessandro%22%2C%22lastName%22%3A%22Donati%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Filippo%22%2C%22lastName%22%3A%22Samperi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Andrea%22%2C%22lastName%22%3A%22Scamporrino%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Maurizio%22%2C%22lastName%22%3A%22Canetti%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matteo%22%2C%22lastName%22%3A%22Mauro%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Francesca%22%2C%22lastName%22%3A%22Villafiorita-Monteleone%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ettore%22%2C%22lastName%22%3A%22Fois%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Chiara%22%2C%22lastName%22%3A%22Botta%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Andrea%22%2C%22lastName%22%3A%22Cappelli%22%7D%5D%2C%22abstractNote%22%3A%22Neutral%20platinum%28II%29%20complex%202-PTPC-BF3a%20was%20easily%20prepared%20from%20benzofulvene%20derivative%202-Pyr-BF3a%20in%20order%20to%20evaluate%20the%20effects%20in%20the%20aggregation%5C%2Fpolymerization%20behavior%20of%20a%20bulky%20substituent%20capable%20of%20establishing%20intermolecular%20metal-metal%20interactions%20in%20the%20close%20proximity%20of%20the%20putative%20polymerization%20center.%20Complex%202-PTPC-BF3a%20was%20found%20to%20aggregate%20into%20an%20ordered%20crystalline%20solid-state%20without%20significant%20spontaneous%20polymerization%2C%20but%20the%20UV-light%20irradiation%20of%20its%20dispersions%20in%20chloroform%20produced%20corresponding%20oligomers%2C%20which%20were%20characterized%20by%20NMR%20spectroscopy%20and%20MALDI-TOF%20mass%20spectrometry.%20On%20the%20other%20hand%2C%20the%20UV-irradiation%20of%20the%20platinum%20complex%20in%20the%20solid-state%20produced%20different%20results%20probably%20depending%20on%20the%20aggregate%20architecture.%20Notably%2C%20the%20crystalline%20films%20deposited%20from%20THE%20solutions%20on%20quartz%20substrates%20showed%20weak%20emissions%2C%20which%20progressively%20increased%20upon%20irradiation%20with%20the%20formation%20of%20oligomers%20devoid%20of%20the%20aggregation-induced%20quenching%20sites%20that%20seemed%20to%20affect%20the%20emission%20of%20poly-2-PTPC-BF3a.%20Finally%2C%20DFT%20calculations%20were%20performed%20on%20platinum%20complex%202-PTPC-BF3a%20in%20the%20aim%20of%20rationalizing%20the%20observed%20photophysical%20features.%22%2C%22date%22%3A%222021%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.eurpolymj.2021.110597%22%2C%22ISSN%22%3A%220014-3057%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1016%5C%2Fj.eurpolymj.2021.110597%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222021-08-17T11%3A54%3A54Z%22%7D%7D%5D%7D
[1]
T. Achard, S. Bellemin-Laponnaz, Recent Advances on Catalytic Osmium-Free Olefin syn-Dihydroxylation, European Journal of Organic Chemistry 2021 (2021) 877–896.
https://doi.org/10.1002/ejoc.202001209 .
[1]
Z. Asbai, A. Bonfiglio, P. Mercandelli, F. Polo, M. Mauro, Cationic rhenium(I) complexes bearing a pi-accepting pyridoannulated N-heterocyclic carbene ligand: Synthesis, photophysical, electrochemical and theoretical investigation, Polyhedron 197 (2021) 115025.
https://doi.org/10.1016/j.poly.2021.115025 .
[1]
A. Bonfiglio, C. McCartin, U. Carrillo, C. Cebrian, P.C. Gros, S. Fournel, A. Kichler, C. Daniel, M. Mauro, Ir-III-Pyridoannelated N-Heterocyclic Carbene Complexes: Potent Theranostic Agents via Mitochondria Targeting, European Journal of Inorganic Chemistry (2021) 1551–1564.
https://doi.org/10.1002/ejic.202100132 .
[1]
H. Chen, Y. Zhang, A. Bonfiglio, F. Morlet-Savary, M. Mauro, J. Lalevee, Rhenium(I) N-Heterocyclic Carbene Complexes in Photoinitiating Systems for Polymerization upon Visible Light: Development of Photosensitive Resins for 3D and 4D Applications, ACS Applied Polymer Materials 3 (2021) 464–473.
https://doi.org/10.1021/acsapm.0c01207 .
[1]
R. De Marco, M. Dal Grande, M. Baron, L. Orian, C. Graiff, T. Achard, S. Bellemin-Laponnaz, A. Pothig, C. Tubaro, Synthesis, Structural Characterization and Antiproliferative Activity of Gold(I) and Gold(III) Complexes Bearing Thioether-Functionalized N-Heterocyclic Carbenes, European Journal of Inorganic Chemistry 2021 (2021) 4196–4206.
https://doi.org/10.1002/ejic.202100495 .
[1]
J. Egly, D. Bissessar, T. Achard, B. Heinrich, P. Steffanut, M. Mauro, S. Bellemin-Laponnaz, Copper(I) complexes with remotely functionalized phosphine ligands: Synthesis, structural variety, photophysics and effect onto the optical properties, Inorganica Chimica Acta 514 (2021) 119971.
https://doi.org/10.1016/j.ica.2020.119971 .
[1]
Y. Geiger, T. Achard, A. Maisse-Francois, S. Bellemin-Laponnaz, Absence of Non-Linear Effects Despite Evidence for Catalyst Aggregation, European Journal of Organic Chemistry (2021) 2916–2922.
https://doi.org/10.1002/ejoc.202100183 .
[1]
F. Hammoud, M. Rahal, J. Egly, F. Morlet-Savary, A. Hijazi, S. Bellemin-Laponnaz, M. Mauro, J. Lalevee, Cubane Cu4I4(phosphine)(4) complexes as new co-initiators for free radical photopolymerization: towards aromatic amine-free systems, Polymer Chemistry 12 (2021) 2848–2859.
https://doi.org/10.1039/d1py00277e .
[1]
S. Le Roux, G. Ori, S. Bellemin-Laponnaz, M. Boero, Tridentate complexes of group 4 bearing bis-aryloxide N-heterocyclic carbene ligand: Structure, spin density and charge states, Chemical Physics Letters 781 (2021) 138888.
https://doi.org/10.1016/j.cplett.2021.138888 .
[1]
M. Marinova, A. Bonnefont, T. Achard, A. Maisse-Francois, S. Bellemin-Laponnaz, Polymerization/depolymerization of chiral metallo-supramolecular assembly induced by redox change, Chirality (2021) 1–8.
https://doi.org/10.1002/chir.23342 .
[1]
M. Mauro, Phosphorescent multinuclear complexes for optoelectronics: tuning of the excited-state dynamics, Chemical Communications (2021) 5857.
https://doi.org/10.1039/d1cc01077h .
[1]
M. Paolino, A. Reale, G. Magrini, V. Razzano, M. Saletti, G. Giuliani, A. Donati, F. Samperi, A. Scamporrino, M. Canetti, M. Mauro, F. Villafiorita-Monteleone, E. Fois, C. Botta, A. Cappelli, Synthesis and UV-light induced oligomerization of a benzofulvene-based neutral platinum(II) complex, European Polymer Journal 156 (2021) 110597.
https://doi.org/10.1016/j.eurpolymj.2021.110597 .
1839302
ITCCYZMF
2020
1
surface-science-reports
50
creator
asc
year
10207
https://www.ipcms.fr/plugins/zotpress/
%7B%22status%22%3A%22success%22%2C%22updateneeded%22%3Afalse%2C%22instance%22%3Afalse%2C%22meta%22%3A%7B%22request_last%22%3A0%2C%22request_next%22%3A0%2C%22used_cache%22%3Atrue%7D%2C%22data%22%3A%5B%7B%22key%22%3A%22RQMITG74%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Achard%20et%20al.%22%2C%22parsedDate%22%3A%222020%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ET.%20Achard%2C%20L.%20Egger%2C%20C.%20Tortoreto%2C%20L.%20Gu%26%23xE9%3Bn%26%23xE9%3Be%2C%20J.%20Lacour%2C%20Preparation%20and%20Structural%20Characterization%20of%20%5BCpRu%281%2C10-phenanthroline%29%28CH3CN%29%5D%5BX%5D%20and%20Precursor%20Complexes%20%28X%3DPF6%2C%20BArF%2C%20TRISPHAT-N%29%2C%20Helvetica%20Chimica%20Acta%20103%20%282020%29%20e2000190.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2Fhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fhlca.202000190%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2Fhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fhlca.202000190%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Preparation%20and%20Structural%20Characterization%20of%20%5BCpRu%281%2C10-phenanthroline%29%28CH3CN%29%5D%5BX%5D%20and%20Precursor%20Complexes%20%28X%3DPF6%2C%20BArF%2C%20TRISPHAT-N%29%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thierry%22%2C%22lastName%22%3A%22Achard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22L%5Cu00e9o%22%2C%22lastName%22%3A%22Egger%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Cecilia%22%2C%22lastName%22%3A%22Tortoreto%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Laure%22%2C%22lastName%22%3A%22Gu%5Cu00e9n%5Cu00e9e%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22J%5Cu00e9r%5Cu00f4me%22%2C%22lastName%22%3A%22Lacour%22%7D%5D%2C%22abstractNote%22%3A%22Abstract%20Cationic%20%5BRu%28%5Cu03b75-C5H5%29%28CH3CN%293%5D%2B%20complex%2C%20tris%28acetonitrile%29%28cyclopentadienyl%29ruthenium%28II%29%2C%20gives%20rise%20to%20a%20very%20rich%20organometallic%20chemistry.%20Combined%20with%20diimine%20ligands%2C%20and%201%2C10-phenanthroline%20in%20particular%2C%20this%20system%20efficiently%20catalyzes%20diazo%20decomposition%20processes%20to%20generate%20metal-carbenes%20which%20undergo%20a%20series%20of%20original%20transformations%20in%20the%20presence%20of%20Lewis%20basic%20substrates.%20Herein%2C%20syntheses%20and%20characterizations%20of%20%5BCpRu%28Phen%29%28L%29%5D%20complexes%20with%20%28large%29%20lipophilic%20non-coordinating%20%28PF6%5Cu2212%20and%20BArF%5Cu2212%29%20and%20coordinating%20TRISPHAT-N%5Cu2212%20anions%20are%20reported.%20Complex%20%5BCpRu%28%5Cu03b76-naphthalene%29%5D%5BBArF%5D%20%28%5B1%5D%5BBArF%5D%29%20is%20readily%20accessible%2C%20in%20high%20yield%2C%20by%20direct%20counterion%20exchange%20between%20%5B1%5D%5BPF6%5D%20and%20sodium%20tetrakis%5B3%2C5-bis%28trifluoromethyl%29phenyl%5Dborate%20%28NaBArF%29%20salts.%20Ligand%20exchange%20of%20%5B1%5D%5BBArF%5D%20in%20acetonitrile%20generated%20stable%20%5BRu%28%5Cu03b75-C5H5%29%28CH3CN%293%5D%5BBArF%5D%20%28%5B2%5D%5BBArF%5D%29%20complex%20in%20high%20yield.%20Then%2C%20the%20desired%20%5BCpRu%28Phen%29%28CH3CN%29%5D%20%28%5B3%5D%29%20complexes%20were%20obtained%20from%20either%20the%20%5B1%5D%20or%20%5B2%5D%20complex%20in%20the%20presence%20of%20the%201%2C10-phenanthroline%20as%20ligand.%20For%20characterization%20and%20comparison%20purposes%2C%20the%20anionic%20hemilabile%20ligand%20TRISPHAT%5Cu2212N%20%28TTN%29%20was%20introduced%20on%20the%20ruthenium%20center%2C%20from%20the%20complex%20%5B3%5D%5BPF6%5D%2C%20to%20quantitatively%20generate%20the%20desired%20complex%20%5BCpRu%28Phen%29%28TTN%29%5D%20%28%5B4%5D%29%20by%20displacement%20of%20the%20remaining%20acetonitrile%20ligand%20and%20of%20the%20PF6%5Cu2212%20anion.%20Solid%20state%20structures%20of%20complexes%20%5B1%5D%5BBArF%5D%2C%20%5B2%5D%5BBArF%5D%2C%20%5B3%5D%5BBArF%5D%2C%20%5B3%5D%5BPF6%5D%20and%20%5B4%5D%20were%20determined%20by%20X-ray%20diffraction%20studies%20and%20are%20discussed%20herein.%22%2C%22date%22%3A%222020%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%22https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fhlca.202000190%22%2C%22ISSN%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fonlinelibrary.wiley.com%5C%2Fdoi%5C%2Fabs%5C%2F10.1002%5C%2Fhlca.202000190%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222022-02-08T08%3A30%3A29Z%22%7D%7D%2C%7B%22key%22%3A%228WV7KMNJ%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Bellemin-Laponnaz%22%2C%22parsedDate%22%3A%222020%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ES.%20Bellemin-Laponnaz%2C%20N-Heterocyclic%20Carbene%20Platinum%20Complexes%3A%20A%20Big%20Step%20Forward%20for%20Effective%20Antitumor%20Compounds%2C%20European%20Journal%20of%20Inorganic%20Chemistry%202020%20%282020%29%2010%26%23x2013%3B20.%20%3Ca%20class%3D%27zp-ItemURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fejic.201900960%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fejic.201900960%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22N-Heterocyclic%20Carbene%20Platinum%20Complexes%3A%20A%20Big%20Step%20Forward%20for%20Effective%20Antitumor%20Compounds%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%5D%2C%22abstractNote%22%3A%22The%20discovery%20of%20cisplatin%20is%20an%20important%20milestone%20in%20the%20history%20of%20successful%20anticancer%20drugs.%20This%20is%20also%20a%20notable%20example%20of%20the%20translational%20scientific%20achievement%20that%20remains%20a%20driving%20force%20in%20multidisciplinary%20research.%20The%20clinical%20success%20of%20cisplatin%20has%20led%20chemists%20to%20vary%20the%20ligands%20around%20the%20platinum%20metal%20to%20improve%20cisplatin%27s%20effectiveness%20while%20reducing%20its%20side%20effects.%20While%20there%20have%20been%20more%20failures%20than%20successes%2C%20key%20developments%20in%20the%20elucidation%20of%20mechanisms%20of%20the%20tumor-resistance%20properties%20have%20been%20accomplished.%20N-Heterocyclic%20carbene%20as%20ligand%20for%20organometallic%20chemistry%20is%20a%20relatively%20young%20area%20that%20offers%20new%20opportunities%20in%20many%20fields%20including%20bio-inorganic%20chemistry.%20These%20ligands%20fit%20the%20prerequisites%20for%20metal%20drug%20development%20and%20recent%20achievements%20have%20demonstrated%20the%20great%20potential%20of%20these%20compounds.%20The%20properties%20of%20these%20metal%20NHC%20complexes%20also%20allow%20for%20easy%20post-functionalization%2C%20thus%20enabling%20molecular%20diversity%20for%20efficient%20drug%20design.%20Overall%2C%20N-heterocyclic%20carbene%20ligands%20reveal%20new%20opportunities%20for%20Pt%20drug%20development.%22%2C%22date%22%3A%222020%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1002%5C%2Fejic.201900960%22%2C%22ISSN%22%3A%221434-1948%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fejic.201900960%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22BMA9GKQT%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222021-04-30T13%3A58%3A48Z%22%7D%7D%2C%7B%22key%22%3A%22964GNIXZ%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Bonfiglio%20and%20Mauro%22%2C%22parsedDate%22%3A%222020%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EA.%20Bonfiglio%2C%20M.%20Mauro%2C%20PhosphorescentTris-Bidentate%20Ir%28III%29Complexes%20with%20N-Heterocyclic%20Carbene%20Scaffolds%3A%20Structural%20Diversity%20and%20Optical%20Properties%2C%20European%20Journal%20of%20Inorganic%20Chemistry%20%282020%29%203427%26%23x2013%3B3442.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fejic.202000509%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fejic.202000509%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22PhosphorescentTris-Bidentate%20Ir%28III%29Complexes%20with%20N-Heterocyclic%20Carbene%20Scaffolds%3A%20Structural%20Diversity%20and%20Optical%20Properties%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Anna%22%2C%22lastName%22%3A%22Bonfiglio%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matteo%22%2C%22lastName%22%3A%22Mauro%22%7D%5D%2C%22abstractNote%22%3A%22N-heterocyclic%20carbenes%20%28NHCs%29%20are%20highly%20versatile%20ligands%20with%20unique%20features%20that%20are%20able%20to%20engage%20into%20robust%20metal-ligand%20bonds%2C%20owing%20to%20their%20strong%20electron%20sigma-donating%20and%20relatively%20weak%20pi-accepting%20abilities.%20Aiming%20at%20efficient%20and%20stable%20emitters%2C%20coordination%20features%20of%20NHCs%20have%20been%20successfully%20employed%20for%20modulating%20both%20optical%20and%20redox%20properties%20yielding%20outstanding%20Ir%28III%29complexes.%20In%20addition%2C%20NHCs%20are%20an%20unparalleled%20tool%20that%20helps%20to%20suppressing%20nonradiative%20pathways%20and%20shift%20emission%20into%20the%20blue%20region.%20As%20a%20result%2C%20Ir%28III%29complexes%20with%20NHC%20ligands%20represent%20an%20appealing%20class%20of%20phosphorescent%20emitters%20that%20show%20great%20potential%20application%20in%20light-emitting%20devices%2C%20electro-chemiluminescence%20and%20biomedicine.%20Herein%2C%20the%20interplay%20between%20structural%20diversity%20and%20photophysical%20properties%20will%20be%20discussed%20for%20a%20colorful%20palette%20of%20Ir-III-NHC%20complexes%20in%20a%20systematic%20approach.%20Finally%2C%20their%20employment%20in%20optoelectronic%20devices%20and%20biomedicine%20will%20be%20described.%22%2C%22date%22%3A%222020%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1002%5C%2Fejic.202000509%22%2C%22ISSN%22%3A%221434-1948%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1002%5C%2Fejic.202000509%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22BMA9GKQT%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222022-02-11T12%3A29%3A54Z%22%7D%7D%2C%7B%22key%22%3A%22QI8G6BXA%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Bonfiglio%20et%20al.%22%2C%22parsedDate%22%3A%222020%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EA.%20Bonfiglio%2C%20L.%20Pallova%2C%20V.%20Cesar%2C%20C.%20Gourlaouen%2C%20S.%20Bellemin-Laponnaz%2C%20C.%20Daniel%2C%20F.%20Polo%2C%20M.%20Mauro%2C%20Phosphorescent%20Cationic%20Heterodinuclear%20Ir-III%5C%2FM%28I%29Complexes%20%28M%3DCu-I%2C%20Au-I%29%20with%20a%20Hybrid%20Janus-Type%20N-Heterocyclic%20Carbene%20Bridge%2C%20Chemistry-a%20European%20Journal%2026%20%282020%29%201%26%23x2013%3B17.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fchem.202002767%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fchem.202002767%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Phosphorescent%20Cationic%20Heterodinuclear%20Ir-III%5C%2FM%28I%29Complexes%20%28M%3DCu-I%2C%20Au-I%29%20with%20a%20Hybrid%20Janus-Type%20N-Heterocyclic%20Carbene%20Bridge%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Anna%22%2C%22lastName%22%3A%22Bonfiglio%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Lenka%22%2C%22lastName%22%3A%22Pallova%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Vincent%22%2C%22lastName%22%3A%22Cesar%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Christophe%22%2C%22lastName%22%3A%22Gourlaouen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Chantal%22%2C%22lastName%22%3A%22Daniel%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Federico%22%2C%22lastName%22%3A%22Polo%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matteo%22%2C%22lastName%22%3A%22Mauro%22%7D%5D%2C%22abstractNote%22%3A%22A%20novel%20class%20of%20phosphorescent%20cationic%20heterobimetallic%20Ir-III%5C%2FM%28I%29complexes%2C%20where%20M-I%3DCu-I%284%29%20and%20Au-I%285%29%2C%20is%20reported.%20The%20two%20metal%20centers%20are%20connected%20by%20the%20hybrid%20bridging%201%2C3-dimesityl-5-acetylimidazol-2-ylidene-4-olate%20%28IMesAcac%29%20ligand%20that%20combines%20both%20a%20chelating%20acetylacetonato-like%20and%20a%20monodentate%20N-heterocyclic%20carbene%20site%20coordinated%20onto%20an%20Ir%28III%29and%20a%20M%28I%29center%2C%20respectively.%20Complexes%204and5have%20been%20prepared%20straightforwardly%20by%20a%20stepwise%20site-selective%20metalation%20with%20the%20zwitterionic%20%5B%28IPr%29M-I%28IMesAcac%29%5D%20metalloproligand%20%28IPr%3D1%2C3-%282%2C6-diisopropylphenyl%29-2H-imidazol-2-ylidene%29%20and%20they%20have%20been%20fully%20characterized%20by%20spectroscopic%2C%20electrochemical%2C%20and%20computational%20investigation.%20Complexes%204and5display%20intense%20red%20emission%20arising%20from%20a%20low-energy%20excited%20state%20that%20is%20located%20onto%20the%20%5Cu201cIr%28C%3C%5E%3EN%29%5Cu201d%20moiety%20featuring%20an%20admixed%20triplet%20ligand-centered%5C%2Fmetal-to-ligand%20charge%20transfer%20%28%28IL%29-I-3%5C%2F%28MLCT%29-M-1%29%20character.%20Comparison%20with%20the%20benchmark%20mononuclear%20complexes%20reveals%20negligible%20electronic%20coupling%20between%20the%20two%20distal%20metal%20centers%20at%20the%20electronic%20ground%20state.%20The%20bimetallic%20systems%20display%20enhanced%20photophysical%20properties%20in%20comparison%20with%20the%20parental%20congeners.%20Noteworthy%2C%20similar%20non-radiative%20rate%20constants%20have%20been%20determined%20along%20with%20a%20two-fold%20increase%20of%20radiative%20rate%2C%20yielding%20brightly%20red-emitting%20cyclometalating%20Ir%28III%29complexes.%20This%20finding%20is%20ascribed%20to%20the%20increased%20MLCT%20character%20of%20the%20emitting%20state%20in%20complexes%204and5due%20to%20the%20smaller%20energy%20gap%20between%20the%283%29IL%20and%281%29MLCT%20manifolds%2C%20which%20mix%20via%20spin-orbit%20coupling.%22%2C%22date%22%3A%222020%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1002%5C%2Fchem.202002767%22%2C%22ISSN%22%3A%220947-6539%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1002%5C%2Fchem.202002767%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22BMA9GKQT%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222021-04-30T13%3A59%3A32Z%22%7D%7D%2C%7B%22key%22%3A%22I6K7SXWK%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Bonfiglio%20et%20al.%22%2C%22parsedDate%22%3A%222020%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EA.%20Bonfiglio%2C%20K.%20Magra%2C%20C.%20Cebrian%2C%20F.%20Polo%2C%20P.C.%20Gros%2C%20P.%20Mercandelli%2C%20M.%20Mauro%2C%20Red-emitting%20neutral%20rhenium%28I%29%20complexes%20bearing%20a%20pyridyl%20pyridoannelated%20N-heterocyclic%20carbene%2C%20Dalton%20Transactions%2049%20%282020%29%203102%26%23x2013%3B3111.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fc9dt04890a%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fc9dt04890a%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Red-emitting%20neutral%20rhenium%28I%29%20complexes%20bearing%20a%20pyridyl%20pyridoannelated%20N-heterocyclic%20carbene%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Anna%22%2C%22lastName%22%3A%22Bonfiglio%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Kevin%22%2C%22lastName%22%3A%22Magra%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Cristina%22%2C%22lastName%22%3A%22Cebrian%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Federico%22%2C%22lastName%22%3A%22Polo%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Philippe%20C.%22%2C%22lastName%22%3A%22Gros%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Pierluigi%22%2C%22lastName%22%3A%22Mercandelli%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matteo%22%2C%22lastName%22%3A%22Mauro%22%7D%5D%2C%22abstractNote%22%3A%22Two%20novel%20rhenium%28I%29%20tricarbonyl%20complexes%20of%20general%20formula%20fac-%5BRe%28N%3C%5E%3EC%3A%29%28CO%29%283%29X%5D%20are%20herein%20presented%2C%20where%20N%3C%5E%3EC%3A%20is%20the%20pyridoannelated%20N-heterocyclic%20carbene%20%28NHC%29%20arising%20from%202-%282-pyridinyl%29%20imidazo%5B1%2C5-a%5Dpyridinium%20hexafluorophosphate%20proligand%2C%20namely%20%5Bpyipy%5DPF6%2C%20and%20X%20being%20Cl%20and%20Br.%20The%20synthetic%20pathway%20is%20a%20one-pot%20reaction%20that%20starts%20from%20the%20azolium%20salt%20as%20the%20NHC%20source%20and%20%5BRe%28CO%29%285%29X%5D%20to%20yield%20the%20desired%20charge-neutral%20fac-%5BRe%28%20pyipy%29%28CO%29%283%29X%5D%20complexes%20%281-2%29.%20Both%20complexes%20were%20thoroughly%20characterized%20by%20spectroscopic%2C%20electrochemical%2C%20theoretical%20investigation%20as%20well%20as%20X-ray%20diffraction%20analysis.%20They%20display%20a%20rather%20similar%20electronic%20absorption%20spectrum%20in%20dilute%20CH2Cl2%20solution%2C%20which%20is%20characterized%20by%20a%20broad%20profile%20extending%20into%20the%20blue%20region.%20This%20lowest-lying%20absorption%20band%20is%20attributed%20to%20a%20transition%20with%20admixed%20metal-to-ligand%20charge%20transfer%20and%20intraligand%20charge%20transfer%20%28%28MLCT%29-M-1%5C%2F%28ILCT%29-I-1%29%20character.%20Degassed%20samples%20of%20the%20complexes%20display%20moderate%20%28Phi%20approximate%20to%201.5%25%29%20and%20long-lived%20%28tau%20%3D%2012.8-13.4%20mu%20s%29%20red%20photoluminescence%20with%20highly%20structured%20profile%20independent%20of%20the%20nature%20of%20the%20ancillary%20halogen%20ligand%20and%20little%20sensitivity%20to%20the%20solvent%20polarity%2C%20highlighting%20the%20markedly%20different%20nature%20of%20the%20emitting%20excited%20state%20in%20comparison%20with%20the%20lowest-lying%20absorption.%20Indeed%2C%20photoluminescence%20is%20ascribed%20to%20a%20long-lived%20excited%20state%20with%20metal-perturbed%20triplet%20ligand-centred%20%28%28LC%29-L-3%29%20character%20as%20supported%20by%20both%20experimental%20and%20density%20functional%20theory%20%28DFT%29%20investigations.%22%2C%22date%22%3A%222020%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1039%5C%2Fc9dt04890a%22%2C%22ISSN%22%3A%221477-9226%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1039%5C%2Fc9dt04890a%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22BMA9GKQT%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222022-01-22T15%3A33%3A57Z%22%7D%7D%2C%7B%22key%22%3A%22NWE4STXN%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Bouche%20et%20al.%22%2C%22parsedDate%22%3A%222020%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EM.%20Bouche%2C%20B.%20Vincent%2C%20T.%20Achard%2C%20S.%20Bellemin-Laponnaz%2C%20N-Heterocyclic%20Carbene%20Platinum%28IV%29%20as%20Metallodrug%20Candidates%3A%20Synthesis%20and%28195%29Pt%20NMR%20Chemical%20Shift%20Trend%2C%20Molecules%2025%20%282020%29%203148.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fmolecules25143148%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fmolecules25143148%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22N-Heterocyclic%20Carbene%20Platinum%28IV%29%20as%20Metallodrug%20Candidates%3A%20Synthesis%20and%28195%29Pt%20NMR%20Chemical%20Shift%20Trend%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Mathilde%22%2C%22lastName%22%3A%22Bouche%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Bruno%22%2C%22lastName%22%3A%22Vincent%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thierry%22%2C%22lastName%22%3A%22Achard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%5D%2C%22abstractNote%22%3A%22A%20series%20of%20octahedral%20platinum%28IV%29%20complexes%20functionalized%20with%20bothN-heterocyclic%20carbene%20%28NHC%29%20ligands%20were%20synthesized%20according%20to%20a%20straightforward%20procedure%20and%20characterized.%20The%20coordination%20sphere%20around%20the%20metal%20was%20varied%2C%20investigating%20the%20influence%20of%20the%20substituted%20NHC%20and%20the%20amine%20ligand%20in%20trans%20position%20to%20the%20NHC.%20The%20influence%20of%20those%20structural%20variations%20on%20the%20chemical%20shift%20of%20the%20platinum%20center%20were%20evaluated%20by%28195%29Pt%20NMR.%20This%20spectroscopy%20provided%20more%20insights%20on%20the%20impact%20of%20the%20structural%20changes%20on%20the%20electronic%20density%20at%20the%20platinum%20center.%20Investigation%20of%20the%20in%20vitro%20cytotoxicities%20of%20representative%20complexes%20were%20carried%20on%20three%20cancer%20cell%20lines%20and%20showed%20IC%2850%29values%20down%20to%20the%20low%20micromolar%20range%20that%20compare%20favorably%20with%20the%20benchmark%20cisplatin%20or%20their%20platinum%28II%29%20counterparts%20bearing%20NHC%20ligands.%22%2C%22date%22%3A%222020%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.3390%5C%2Fmolecules25143148%22%2C%22ISSN%22%3A%22%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.3390%5C%2Fmolecules25143148%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22BMA9GKQT%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222021-04-30T13%3A55%3A47Z%22%7D%7D%2C%7B%22key%22%3A%22PGJYRPMS%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Charignon%20et%20al.%22%2C%22parsedDate%22%3A%222020%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EE.%20Charignon%2C%20M.%20Bouche%2C%20C.%20Clave-Darcissac%2C%20G.%20Dahm%2C%20G.%20Ichim%2C%20A.%20Clotagatide%2C%20H.C.%20Mertani%2C%20P.%20Telouk%2C%20J.%20Caramel%2C%20J.-J.%20Diaz%2C%20S.%20Bellemin-Laponnaz%2C%20P.%20Bouvet%2C%20C.%20Billotey%2C%20In%20Cellulo%20Evaluation%20of%20the%20Therapeutic%20Potential%20of%20NHC%20Platinum%20Compounds%20in%20Metastatic%20Cutaneous%20Melanoma%2C%20International%20Journal%20of%20Molecular%20Sciences%2021%20%282020%29%207626.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fijms21217826%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fijms21217826%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22In%20Cellulo%20Evaluation%20of%20the%20Therapeutic%20Potential%20of%20NHC%20Platinum%20Compounds%20in%20Metastatic%20Cutaneous%20Melanoma%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Elsa%22%2C%22lastName%22%3A%22Charignon%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Mathilde%22%2C%22lastName%22%3A%22Bouche%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Caroline%22%2C%22lastName%22%3A%22Clave-Darcissac%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Georges%22%2C%22lastName%22%3A%22Dahm%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Gabriel%22%2C%22lastName%22%3A%22Ichim%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Anthony%22%2C%22lastName%22%3A%22Clotagatide%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Hichem%20C.%22%2C%22lastName%22%3A%22Mertani%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Philippe%22%2C%22lastName%22%3A%22Telouk%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Julie%22%2C%22lastName%22%3A%22Caramel%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jean-Jacques%22%2C%22lastName%22%3A%22Diaz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Philippe%22%2C%22lastName%22%3A%22Bouvet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Claire%22%2C%22lastName%22%3A%22Billotey%22%7D%5D%2C%22abstractNote%22%3A%22We%20describe%20here%20the%20evaluation%20of%20the%20cytotoxic%20efficacy%20of%20two%20platinum%20%28II%29%20complexes%20bearing%20an%20N-heterocyclic%20carbene%20%28NHC%29%20ligand%2C%20a%20pyridine%20ligand%20and%20bromide%20or%20iodide%20ligands%20on%20a%20panel%20of%20human%20metastatic%20cutaneous%20melanoma%20cell%20lines%20representing%20different%20genetic%20subsets%20including%20BRAF-inhibitor-resistant%20cell%20lines%2C%20namely%20A375%2C%20SK-MEL-28%2C%20MeWo%2C%20HMCB%2C%20A375-R%2C%20SK-MEL-5-R%20and%20501MEL-R.%20Cisplatin%20and%20dacarbazine%20were%20also%20studied%20for%20comparison%20purposes.%20Remarkably%2C%20the%20iodine-labelled%20Pt-NHC%20complex%20strongly%20inhibited%20proliferation%20of%20all%20tested%20melanoma%20cells%20after%201-h%20exposure%2C%20likely%20due%20to%20its%20rapid%20uptake%20by%20melanoma%20cells.%20The%20mechanism%20of%20this%20inhibitory%20activity%20involves%20the%20formation%20of%20DNA%20double-strand%20breaks%20and%20apoptosis.%20Considering%20the%20intrinsic%20chemoresistance%20of%20metastatic%20melanoma%20cells%20of%20current%20systemic%20treatments%2C%20these%20findings%20are%20promising%20and%20could%20give%20research%20opportunities%20in%20the%20future%20to%20improve%20the%20prognosis%20of%20patients%20suffering%20from%20unresectable%20metastatic%20melanoma%20that%20are%20not%20eligible%20or%20that%20do%20not%20respond%20to%20the%20most%20effective%20drugs%20available%20to%20date%2C%20namely%20BRAF%20inhibitors%20and%20the%20anti-PD-1%20monoclonal%20antibody%20%28mAb%29.%22%2C%22date%22%3A%222020%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.3390%5C%2Fijms21217826%22%2C%22ISSN%22%3A%22%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.3390%5C%2Fijms21217826%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22BMA9GKQT%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222021-04-30T14%3A01%3A56Z%22%7D%7D%2C%7B%22key%22%3A%22ZE4WAKXG%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Chen%20et%20al.%22%2C%22parsedDate%22%3A%222020%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EW.%20Chen%2C%20J.%20Egly%2C%20A.%20Pobtador-Bahamonde%20I.%2C%20A.%20Maisse-Francois%2C%20S.%20Bellemin-Laponnaz%2C%20T.%20Achard%2C%20Synthesis%2C%20characterization%2C%20catalytic%20and%20biological%20application%20of%20half-sandwich%20ruthenium%20complexes%20bearing%20hemilabile%20%28kappa%202-C%2CS%29-thioether-functionalised%20NHC%20ligands%2C%20Dalton%20Transactions%2049%20%282020%29%203243%26%23x2013%3B3252.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fc9dt04825a%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fc9dt04825a%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Synthesis%2C%20characterization%2C%20catalytic%20and%20biological%20application%20of%20half-sandwich%20ruthenium%20complexes%20bearing%20hemilabile%20%28kappa%202-C%2CS%29-thioether-functionalised%20NHC%20ligands%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Weiguang%22%2C%22lastName%22%3A%22Chen%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Julien%22%2C%22lastName%22%3A%22Egly%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Amatia%2C%20I%22%2C%22lastName%22%3A%22Pobtador-Bahamonde%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Aline%22%2C%22lastName%22%3A%22Maisse-Francois%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thierry%22%2C%22lastName%22%3A%22Achard%22%7D%5D%2C%22abstractNote%22%3A%22A%20series%20of%20cationic%20Ru%28ii%29%28eta%286%29-p-cymene%29%20complexes%20with%20thioether-functionalised%20N-heterocyclic%20carbene%20ligands%20have%20been%20prepared%20and%20fully%20characterized.%20Steric%20and%20electronic%20influence%20of%20the%20R%20thioether%20substituent%20on%20the%20coordination%20of%20the%20sulfur%20atom%20was%20investigated.%20The%20molecular%20structure%20of%20three%20of%20them%20has%20been%20determined%20by%20means%20of%20X-ray%20diffractrometry%20and%20confirmed%20the%20bidentate%20%28kappa%282%29-C%2CS%29%20coordination%20mode%20of%20the%20ligand.%20Interestingly%2C%20only%20a%20single%20diastereomer%2C%20as%20an%20enantiomeric%20couple%2C%20was%20observed%20in%20the%20solid%20state%20for%20complexes%201c%2C%201i%20and%201j.%20DFT%20calculations%20established%20a%20low%20energy%20inversion%20barrier%20between%20the%20two%20diastereomers%20through%20a%20sulfur%20pyramidal%20inversion%20pathway%20with%20R%20donating%20group%20while%20a%20dissociative%5C%2Fassociative%20mechanism%20is%20more%20likely%20with%20R%20substituents%20that%20contain%20electron%20withdrawing%20group%2C%20thus%20suggesting%20that%20the%20only%20species%20observed%20by%20the%20H-1-NMR%20correspond%20to%20an%20average%20resonance%20position%20of%20a%20fluxional%20mixtures%20of%20isomers.%20All%20these%20complexes%20were%20found%20to%20catalyse%20the%20oxydant-free%20double%20dehydrogenation%20of%20primary%20amine%20into%20nitrile.%20Ru%20complex%20bearing%20NHC-functionalised%20S-tBu%20group%20was%20further%20investigated%20in%20a%20wide%20range%20of%20amines%20and%20was%20found%20more%20selective%20for%20alkyl%20amine%20substrates%20than%20for%20benzylamine%20derivatives.%20Finally%2C%20preliminary%20results%20of%20the%20biological%20effects%20on%20various%20human%20cancer%20cells%20of%20four%20selected%20Ru%20complexes%20are%20reported.%22%2C%22date%22%3A%222020%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1039%5C%2Fc9dt04825a%22%2C%22ISSN%22%3A%221477-9226%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1039%5C%2Fc9dt04825a%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22BMA9GKQT%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222022-01-22T15%3A36%3A07Z%22%7D%7D%2C%7B%22key%22%3A%22CPJ3RCDG%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Geiger%20et%20al.%22%2C%22parsedDate%22%3A%222020%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EY.%20Geiger%2C%20T.%20Achard%2C%20A.%20Maisse-Francois%2C%20S.%20Bellemin-Laponnaz%2C%20Observation%20of%20hyperpositive%20non-linear%20effect%20in%20catalytic%20asymmetric%20organozinc%20additions%20to%20aldehydes%2C%20Chirality%20%282020%29%201%26%23x2013%3B7.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fchir.23271%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fchir.23271%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Observation%20of%20hyperpositive%20non-linear%20effect%20in%20catalytic%20asymmetric%20organozinc%20additions%20to%20aldehydes%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yannick%22%2C%22lastName%22%3A%22Geiger%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thierry%22%2C%22lastName%22%3A%22Achard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Aline%22%2C%22lastName%22%3A%22Maisse-Francois%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%5D%2C%22abstractNote%22%3A%22Asymmetric%20amplification%20is%20a%20phenomenon%20that%20is%20believed%20to%20play%20a%20key%20role%20in%20the%20emergence%20of%20homochirality%20in%20life.%20In%20asymmetric%20catalysis%2C%20theoretical%20and%20experimental%20models%20have%20been%20investigated%20to%20provide%20an%20understanding%20of%20how%20chiral%20amplification%20is%20possible%2C%20in%20particular%20based%20on%20non-linear%20effects.%20Interestingly%2C%20it%20has%20been%20proposed%20a%20quarter%20century%20ago%20that%20chiral%20catalysts%2C%20when%20not%20enantiopure%20might%20even%20be%20more%20enantioselective%20than%20their%20enantiopure%20counterparts.%20We%20show%20here%20that%20such%20hyperpositive%20non-linear%20effect%20in%20asymmetric%20catalysis%20is%20indeed%20possible.%20An%20in-depth%20study%20into%20the%20underlying%20mechanism%20was%20carried%20out%2C%20and%20the%20scheme%20we%20derive%20differs%20from%20the%20previous%20proposed%20models.%22%2C%22date%22%3A%222020%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1002%5C%2Fchir.23271%22%2C%22ISSN%22%3A%220899-0042%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1002%5C%2Fchir.23271%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22BMA9GKQT%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222021-04-30T13%3A56%3A40Z%22%7D%7D%2C%7B%22key%22%3A%22VMX7W7DM%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Geiger%20et%20al.%22%2C%22parsedDate%22%3A%222020%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EY.%20Geiger%2C%20T.%20Achard%2C%20A.%20Maisse-Francois%2C%20S.%20Bellemin-Laponnaz%2C%20Hyperpositive%20non-linear%20effects%3A%20enantiodivergence%20and%20modelling%2C%20Chemical%20Science%2011%20%282020%29%2012453%26%23x2013%3B12463.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fd0sc04724d%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fd0sc04724d%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Hyperpositive%20non-linear%20effects%3A%20enantiodivergence%20and%20modelling%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yannick%22%2C%22lastName%22%3A%22Geiger%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thierry%22%2C%22lastName%22%3A%22Achard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Aline%22%2C%22lastName%22%3A%22Maisse-Francois%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%5D%2C%22abstractNote%22%3A%22The%20chiral%20ligand%20N-methylephedrine%20%28NME%29%20was%20found%20to%20catalyse%20the%20addition%20of%20dimethylzinc%20to%20benzaldehyde%20in%20an%20enantiodivergent%20way%2C%20with%20a%20monomeric%20and%20a%20homochiral%20dimeric%20complex%20both%20catalysing%20the%20reaction%20at%20a%20steady%20state%20and%20giving%20opposite%20product%20enantiomers.%20A%20change%20in%20the%20sign%20of%20the%20enantiomeric%20product%20was%20thus%20possible%20by%20simply%20varying%20the%20catalyst%20loading%20or%20the%20ligand%20ee%2C%20giving%20rise%20to%20an%20enantiodivergent%20non-linear%20effect.%20Simulations%20using%20a%20mathematical%20model%20confirmed%20the%20possibility%20of%20such%20behaviour%20and%20showed%20that%20this%20can%20lead%20to%20situations%20where%20a%20reaction%20gives%20racemic%20products%2C%20although%20the%20system%20is%20composed%20only%20of%20highly%20enantioselective%20individual%20catalysts.%20Furthermore%2C%20depending%20on%20the%20dimer%27s%20degree%20of%20participation%20in%20the%20catalytic%20conversion%2C%20enantiodivergence%20may%20or%20may%20not%20be%20observed%20experimentally%2C%20which%20raises%20questions%20about%20the%20possibility%20of%20enantiodivergence%20in%20other%20monomer%5C%2Fdimer-catalysed%20systems.%20Simulations%20of%20the%20reaction%20kinetics%20showed%20that%20the%20observed%20kinetic%20constant%20k%28obs%29%20is%20highly%20dependent%20on%20user-controlled%20parameters%2C%20such%20as%20the%20catalyst%20concentration%20and%20the%20ligand%20ee%2C%20and%20may%20thus%20vary%20in%20a%20distinct%20way%20from%20one%20experimental%20setup%20to%20another.%20This%20unusual%20dependency%20of%20k%28obs%29%20allowed%20us%20to%20confirm%20that%20a%20previously%20observed%20U-shaped%20catalyst%20order%20vs.%20catalyst%20loading-plot%20is%20linked%20to%20the%20simultaneous%20catalytic%20activity%20of%20both%20monomeric%20and%20dimeric%20complexes.%22%2C%22date%22%3A%222020%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1039%5C%2Fd0sc04724d%22%2C%22ISSN%22%3A%222041-6520%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1039%5C%2Fd0sc04724d%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22BMA9GKQT%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222021-04-30T13%3A56%3A16Z%22%7D%7D%2C%7B%22key%22%3A%22QB3VW7RA%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Geiger%20et%20al.%22%2C%22parsedDate%22%3A%222020%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EY.%20Geiger%2C%20T.%20Achard%2C%20A.%20Maisse-Francois%2C%20S.%20Bellemin-Laponnaz%2C%20Hyperpositive%20nonlinear%20effects%20in%20asymmetric%20catalysis%2C%20Nature%20Catalysis%20%282020%29.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1038%5C%2Fs41929-020-0441-1%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1038%5C%2Fs41929-020-0441-1%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Hyperpositive%20nonlinear%20effects%20in%20asymmetric%20catalysis%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yannick%22%2C%22lastName%22%3A%22Geiger%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thierry%22%2C%22lastName%22%3A%22Achard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Aline%22%2C%22lastName%22%3A%22Maisse-Francois%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%5D%2C%22abstractNote%22%3A%22Asymmetric%20amplification%20is%20a%20curious%20phenomenon%20that%20is%20believed%20to%20play%20a%20key%20role%20in%20the%20emergence%20of%20biological%20homochirality%2C%20and%20thus%20of%20life%20itself.%20In%20asymmetric%20catalysis%2C%20it%20is%20achieved%20via%20positive%20nonlinear%20effects%2C%20which%20allow%20high%20product%20enantiomeric%20excesses%20with%20a%20non-enantiopure%20catalyst.%20However%2C%20it%20has%20also%20been%20proposed%20that%20non-enantiopure%20catalysts%20may%20be%20even%20more%20enantioselective%20than%20their%20enantiopure%20counterparts%2C%20although%20such%20a%20case%20has%20never%20been%20experimentally%20observed%20so%20far.%20Here%2C%20we%20present%20an%20example%20of%20such%20a%20hyperpositive%20nonlinear%20effect%20in%20asymmetric%20catalysis.%20We%20found%20that%20addition%20of%20dialkylzinc%20reagents%20to%20benzaldehyde%20gave%20higher%20product%20enantiomeric%20excesses%20with%20only%20partially%20resolved%20chiral%20N-benzyl-ephedrine%20ligands.%20A%20mechanistic%20study%20was%20carried%20out%20and%20our%20results%20point%20towards%20a%20two-component%20catalysis%2C%20where%20mononuclear%20as%20well%20as%20aggregated%20catalysts%20are%20in%20equilibrium%20and%20in%20competition.%20These%20results%20introduce%20an%20unprecedented%20class%20of%20asymmetric%20amplification%20in%20enantioselective%20catalysis.%22%2C%22date%22%3A%222020%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1038%5C%2Fs41929-020-0441-1%22%2C%22ISSN%22%3A%222520-1158%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1038%5C%2Fs41929-020-0441-1%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22BMA9GKQT%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222022-01-22T15%3A40%3A37Z%22%7D%7D%2C%7B%22key%22%3A%22384VWS3J%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Kumar%20et%20al.%22%2C%22parsedDate%22%3A%222020%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EK.S.%20Kumar%2C%20I.%20Salitros%2C%20B.%20Heinrich%2C%20S.%20Moldovan%2C%20M.%20Mauro%2C%20M.%20Ruben%2C%20Spin-crossover%20in%20iron%28II%29-phenylene%20ethynylene-2%2C6-di%28pyrazol-1-yl%29%20pyridine%20hybrids%3A%20toward%20switchable%20molecular%20wire-like%20architectures%2C%20Journal%20of%20Physics-Condensed%20Matter%2032%20%282020%29%20204002.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1088%5C%2F1361-648X%5C%2Fab6cc2%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1088%5C%2F1361-648X%5C%2Fab6cc2%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Spin-crossover%20in%20iron%28II%29-phenylene%20ethynylene-2%2C6-di%28pyrazol-1-yl%29%20pyridine%20hybrids%3A%20toward%20switchable%20molecular%20wire-like%20architectures%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Kuppusamy%20Senthil%22%2C%22lastName%22%3A%22Kumar%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ivan%22%2C%22lastName%22%3A%22Salitros%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Benoit%22%2C%22lastName%22%3A%22Heinrich%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Simona%22%2C%22lastName%22%3A%22Moldovan%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matteo%22%2C%22lastName%22%3A%22Mauro%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Mario%22%2C%22lastName%22%3A%22Ruben%22%7D%5D%2C%22abstractNote%22%3A%22Luminescent%20oligo%28p-phenylene%20ethynylene%29%20%28OPE%29%20and%20spin-crossover%20%28SCO%29%20active%20Fe%28II%292%2C6-di%28pyrazol-1-yl%29%20pyridine%20%28BPP%29%20systems%20are%20prominent%20examples%20proposed%20to%20develop%20functional%20materials%20such%20as%20molecular%20wires%5C%2Fmemories.%20A%20marriage%20between%20OPE%20and%20Fe%28II%29-BPP%20systems%20is%20a%20strategy%20to%20obtain%20supramolecular%20luminescent%20ligands%20capable%20of%20metal%20coordination%20useful%20to%20produce%20novel%20spin-switchable%20hybrids%20with%20synergistic%20coupling%20between%20spin-state%20of%20Fe%28II%29%20and%20a%20physical%20property%20associated%20with%20the%20OPE%20skeleton%2C%20for%20example%2C%20electronic%20conductivity%20or%20luminescence.%20To%20begin%20in%20this%20direction%2C%20two%20novel%20ditopic%20ligands%2C%20namely%20L-1%20and%20L-2%2C%20featuring%20OPE-type%20backbone%20end-capped%20with%20metal%20coordinating%20BPP%20were%20designed%20and%20synthetized.%20The%20ligand%20L-2%20tailored%20with%202-ethylhexyloxy%20chains%20at%20the%202%20and%205%20positions%20of%20the%20OPE%20skeleton%20shows%20modulated%20optical%20properties%20and%20improved%20solubility%20in%20common%20organic%20solvents%20relative%20to%20the%20parent%20ligand%20L-1.%20Solution%20phase%20complexation%20of%20L-1%20and%20L-2%20with%20Fe%28BF4%29%282%29center%20dot%206H%282%29O%20resulted%20in%20the%20formation%20of%20insoluble%20materials%20of%20the%20composition%20%5BFe%28L-1%29%5D%28n%29%28BF4%29%282n%29%20and%20%5BFe%28L-2%29%5D%28n%29%28BF4%29%282n%29%20as%20inferred%20from%20elemental%20analyses.%20Complex%20%5BFe%28L-1%29%5D%28n%29%28BF4%29%282n%29%20underwent%20thermal%20SCO%20centred%20at%20T-1%5C%2F2%20%3D%20275%20K%20as%20well%20as%20photoinduced%20low-spin%20to%20high-spin%20transition%20with%20the%20existence%20of%20the%20metastable%20high-spin%20state%20up%20to%2052%20K.%20On%20the%20other%20hand%2C%20complex%20%5BFe%28L-2%29%5Dn%28BF4%29%282n%29%2C%20tethered%20with%202-ethylhexyloxy%20groups%2C%20showed%20gradual%20and%20half-complete%20SCO%20with%2050%25%20of%20the%20Fe%28II%29-centres%20permanently%20blocked%20in%20the%20high-spin%20state%20due%20to%20intermolecular%20steric%20interactions.%20The%20small%20angle%20x-ray%20scattering%20%28SAXS%29%20pattern%20of%20the%20as-prepared%20solid%20complex%20%5BFe%28L-1%29%5D%28n%29%28BF4%29%282n%29%20revealed%20the%20presence%20of%20nm-sized%20crystallites%20implying%20a%20possible%20methodology%20towards%20the%20template-free%20synthesis%20of%20functional-SCO%20nanostructures.%22%2C%22date%22%3A%222020%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1088%5C%2F1361-648X%5C%2Fab6cc2%22%2C%22ISSN%22%3A%220953-8984%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1088%5C%2F1361-648X%5C%2Fab6cc2%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22TK3HH32E%22%2C%22BMA9GKQT%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222021-04-30T14%3A11%3A05Z%22%7D%7D%2C%7B%22key%22%3A%22M7RJ47ZH%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Marinova%20et%20al.%22%2C%22parsedDate%22%3A%222020%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EM.%20Marinova%2C%20A.%20Bonnefont%2C%20T.%20Achard%2C%20A.%20Maisse-Francois%2C%20S.%20Bellemin-Laponnaz%2C%20Chiral%20stimuli-responsive%20metallo-supramolecular%20assembly%20induced%20by%20Cu-II%5C%2FCu%28I%29redox%20change%2C%20Chemical%20Communications%2056%20%282020%29%208703%26%23x2013%3B8706.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fd0cc01716g%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fd0cc01716g%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Chiral%20stimuli-responsive%20metallo-supramolecular%20assembly%20induced%20by%20Cu-II%5C%2FCu%28I%29redox%20change%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Maya%22%2C%22lastName%22%3A%22Marinova%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Antoine%22%2C%22lastName%22%3A%22Bonnefont%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thierry%22%2C%22lastName%22%3A%22Achard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Aline%22%2C%22lastName%22%3A%22Maisse-Francois%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%5D%2C%22abstractNote%22%3A%22We%20investigated%20the%20selective%20formation%20of%20homoleptic%20and%20heteroleptic%20metal%20complexes%20controlled%20by%20means%20of%20the%20chiral%20molecular%20instruction%20of%20the%20ligand%20and%20the%20coordination%20geometry%20of%20the%20metal.%20Our%20results%20showed%20that%20chiral%20self-recognition%20or%20self-discrimination%20may%20be%20induced%20by%20the%20Cu-I%5C%2FCu%28II%29redox%20transition%20using%20cyclic%20voltammetry.%20The%20further%20use%20of%20chiral%20ditopic%20ligands%20led%20to%20metallo-supramolecular%20copolymers%20with%20stimuli-responsive%20controlled%20arrangement.%22%2C%22date%22%3A%222020%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1039%5C%2Fd0cc01716g%22%2C%22ISSN%22%3A%221359-7345%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1039%5C%2Fd0cc01716g%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22BMA9GKQT%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222021-04-30T13%3A56%3A54Z%22%7D%7D%2C%7B%22key%22%3A%22AAQKIKIA%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Paolino%20et%20al.%22%2C%22parsedDate%22%3A%222020%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EM.%20Paolino%2C%20A.%20Reale%2C%20G.%20Magrini%2C%20V.%20Razzano%2C%20G.%20Giuliani%2C%20A.%20Donati%2C%20G.%20Giorgi%2C%20F.%20Samperi%2C%20M.%20Canetti%2C%20M.%20Mauro%2C%20F.%20Villafiorita-Monteleone%2C%20E.%20Fois%2C%20C.%20Botta%2C%20A.%20Cappelli%2C%20UV-light-induced%20polymerization%20in%20the%20amorphous%20solid-state%20of%20a%20spontaneously%20non-polymerizing%203-phenylbenzofulvene%20monomer%2C%20European%20Polymer%20Journal%20137%20%282020%29%20109923.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.eurpolymj.2020.109923%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.eurpolymj.2020.109923%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22UV-light-induced%20polymerization%20in%20the%20amorphous%20solid-state%20of%20a%20spontaneously%20non-polymerizing%203-phenylbenzofulvene%20monomer%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Marco%22%2C%22lastName%22%3A%22Paolino%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Annalisa%22%2C%22lastName%22%3A%22Reale%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Giulia%22%2C%22lastName%22%3A%22Magrini%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Vincenzo%22%2C%22lastName%22%3A%22Razzano%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Germano%22%2C%22lastName%22%3A%22Giuliani%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Alessandro%22%2C%22lastName%22%3A%22Donati%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Gianluca%22%2C%22lastName%22%3A%22Giorgi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Filippo%22%2C%22lastName%22%3A%22Samperi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Maurizio%22%2C%22lastName%22%3A%22Canetti%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matteo%22%2C%22lastName%22%3A%22Mauro%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Francesca%22%2C%22lastName%22%3A%22Villafiorita-Monteleone%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ettore%22%2C%22lastName%22%3A%22Fois%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Chiara%22%2C%22lastName%22%3A%22Botta%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Andrea%22%2C%22lastName%22%3A%22Cappelli%22%7D%5D%2C%22abstractNote%22%3A%22Benzofulvene%20derivative%202-Pyr-BF3a%20was%20designed%20and%20synthesized%20to%20evaluate%20the%20effects%20of%20a%20pyridine%20ring%20in%20position%202%20of%20the%203-phenylbenzofulvene%20moiety%20on%20the%20spontaneous%20solid-state%20polymerization.%20The%20monomer%20was%20found%20to%20organize%20into%20an%20ordered%20crystalline%20solid-state%20without%20significant%20spontaneous%20polymerization%2C%20but%20the%20irradiation%20of%20amorphous%20film%20samples%20with%20UV-light%20produced%20photopolymerization.%20Resulting%20poly-2-Pyr-BF3a%20was%20characterized%20by%20NMR%20spectroscopy%2C%20MALDI-TOF%20mass%20spectrometry%2C%20and%20photophysical%20studies%20in%20comparison%20with%20the%20corresponding%20monomer.%20Interestingly%2C%20monomer%202-Pyr-BF3a%20was%20weakly%20emissive%20in%20diluted%20solutions%2C%20but%20increased%20its%20PLQY%20in%20the%20crystalline%20solid.%20Conversely%2C%20after%20photopolymerization%20the%20monomeric%20unit%20in%20the%20polymer%20enhanced%20its%20emission%20intensity%20in%20solution%20while%20in%20the%20solid%20state%20results%20weakly%20emissive.%20Even%20more%20interestingly%2C%20this%20benzofulvene%20monomer%20and%20its%20synthetic%20precursors%202%20and%203%20showed%20long-lived%20emission%20suggesting%20a%20phosphorescence%20nature%20of%20the%20emission%20process.%20Finally%2C%20DFT%20and%20TDDFT%20calculations%20were%20performed%20in%20order%20to%20rationalize%20the%20experimental%20data.%22%2C%22date%22%3A%222020%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.eurpolymj.2020.109923%22%2C%22ISSN%22%3A%220014-3057%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1016%5C%2Fj.eurpolymj.2020.109923%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22BMA9GKQT%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222021-04-30T14%3A11%3A13Z%22%7D%7D%2C%7B%22key%22%3A%22CCG7XDAJ%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Romain%20et%20al.%22%2C%22parsedDate%22%3A%222020%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EC.%20Romain%2C%20S.%20Bellemin-Laponnaz%2C%20S.%20Dagorne%2C%20Recent%20progress%20on%20NHC-stabilized%20early%20transition%20metal%20%28group%203-7%29%20complexes%3A%20Synthesis%20and%20applications%2C%20Coordination%20Chemistry%20Reviews%20422%20%282020%29%20213411.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.ccr.2020.213411%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.ccr.2020.213411%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Recent%20progress%20on%20NHC-stabilized%20early%20transition%20metal%20%28group%203-7%29%20complexes%3A%20Synthesis%20and%20applications%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Charles%22%2C%22lastName%22%3A%22Romain%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Samuel%22%2C%22lastName%22%3A%22Dagorne%22%7D%5D%2C%22abstractNote%22%3A%22The%20present%20paper%20comprehensively%20reviews%20group%203-7%20metal%20NHC%20complexes%20reported%20since%202014%2C%20with%20emphasis%20on%20their%20current%20applications%20and%20fundamental%20reactivity.%20Thanks%20to%20NHC%20stabilization%2C%20such%20metal%20complexes%20have%20witnessed%20significant%20and%20diversified%20developments%2C%20essentially%20in%20the%20area%20of%20homogeneous%20catalysis.%20Catalytic%20applications%20primarily%20include%20their%20use%20in%20unsaturated%5C%2Fpolar%20monomer%20polymerization%20catalysis%2C%20hydro-elementation%20of%20C%40O%20and%20C%40N%20substrates%2C%20metathesis-based%20catalytic%20reactions%2C%20hydro-elementation%20of%20C%40O%20substrates%20and%20oxidation%20reactions.%20Apart%20from%20additional%20stability%2C%20the%20use%20of%20NHC%20fine-tuned%20steric%5C%2Felectronic%20properties%20was%20demonstrated%20to%20be%20crucial%20for%20catalytic%20performances.%20NHC-bearing%20Group%206%20and%207%20complexes%2C%20which%20have%20been%20most%20studied%20recently%2C%20have%20also%20been%20exploited%20as%20luminescent%20molecules%20and%20for%20biomedical%20applications%20%28as%20radiopharmaceutical%20and%20theranostic%20agents%29.%20The%20large%20pool%20of%20available%20NHCs%20should%20further%20promote%20developments%20and%20application%20of%20such%20metal%20complexes%20in%20future%20years.%20%28C%29%202020%20Elsevier%20B.V.%20All%20rights%20reserved.%22%2C%22date%22%3A%222020%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.ccr.2020.213411%22%2C%22ISSN%22%3A%220010-8545%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1016%5C%2Fj.ccr.2020.213411%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22BMA9GKQT%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222021-04-30T14%3A04%3A53Z%22%7D%7D%2C%7B%22key%22%3A%22N9EWW7G2%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Verron%20et%20al.%22%2C%22parsedDate%22%3A%222020%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ER.%20Verron%2C%20T.%20Achard%2C%20C.%20Seguin%2C%20S.%20Fournel%2C%20S.%20Bellemin-Laponnaz%2C%20Synthesis%20and%20Characterization%20of%20N-Heterocyclic%20Carbene%20Dithiocarbamate%20Platinum%20Complexes%20with%20Antitumoral%20Activity%2C%20European%20Journal%20of%20Inorganic%20Chemistry%202020%20%282020%29%202552%26%23x2013%3B2557.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fejic.202000329%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fejic.202000329%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Synthesis%20and%20Characterization%20of%20N-Heterocyclic%20Carbene%20Dithiocarbamate%20Platinum%20Complexes%20with%20Antitumoral%20Activity%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Raphael%22%2C%22lastName%22%3A%22Verron%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thierry%22%2C%22lastName%22%3A%22Achard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Cendrine%22%2C%22lastName%22%3A%22Seguin%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sylvie%22%2C%22lastName%22%3A%22Fournel%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22Bellemin-Laponnaz%22%7D%5D%2C%22abstractNote%22%3A%22A%20series%20of%20platinum%28II%29%20complexes%20bearing%20N-heterocyclic%20carbene%20NHC%20of%20the%20type%20%5B%28NHC%29PtX%28L%29%5D%20where%20L%20is%20a%20dithiocarbamate%20ligand%20and%20X%20iodide%20were%20prepared%20and%20fully%20characterized.%20The%20molecular%20structure%20of%20one%20derivative%20was%20determined%20by%20single-crystal%20X-ray%20analyses%20confirming%20that%20the%20dithiocarbamate%20is%20acting%20as%20a%20bidentate%20ligand%20to%20the%20metal.%20All%20complexes%20demonstrated%20potent%20in%20vitro%20antiproliferative%20activities%20against%20cancer%20cells.%20Mechanistic%20investigations%20revealed%20that%20mitochondrial%20dysfunction%20and%20ROS%20%28Reactive%20Oxygen%20Species%29%20production%20were%20correlated%20with%20the%20cytotoxic%20process%20induced%20by%20these%20complexes.%22%2C%22date%22%3A%222020%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1002%5C%2Fejic.202000329%22%2C%22ISSN%22%3A%221434-1948%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1002%5C%2Fejic.202000329%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22BMA9GKQT%22%2C%22ITCCYZMF%22%5D%2C%22dateModified%22%3A%222025-05-27T12%3A46%3A37Z%22%7D%7D%5D%7D
[1]
T. Achard, L. Egger, C. Tortoreto, L. Guénée, J. Lacour, Preparation and Structural Characterization of [CpRu(1,10-phenanthroline)(CH3CN)][X] and Precursor Complexes (X=PF6, BArF, TRISPHAT-N), Helvetica Chimica Acta 103 (2020) e2000190.
https://doi.org/https://doi.org/10.1002/hlca.202000190 .
[1]
S. Bellemin-Laponnaz, N-Heterocyclic Carbene Platinum Complexes: A Big Step Forward for Effective Antitumor Compounds, European Journal of Inorganic Chemistry 2020 (2020) 10–20.
https://doi.org/10.1002/ejic.201900960 .
[1]
A. Bonfiglio, M. Mauro, PhosphorescentTris-Bidentate Ir(III)Complexes with N-Heterocyclic Carbene Scaffolds: Structural Diversity and Optical Properties, European Journal of Inorganic Chemistry (2020) 3427–3442.
https://doi.org/10.1002/ejic.202000509 .
[1]
A. Bonfiglio, L. Pallova, V. Cesar, C. Gourlaouen, S. Bellemin-Laponnaz, C. Daniel, F. Polo, M. Mauro, Phosphorescent Cationic Heterodinuclear Ir-III/M(I)Complexes (M=Cu-I, Au-I) with a Hybrid Janus-Type N-Heterocyclic Carbene Bridge, Chemistry-a European Journal 26 (2020) 1–17.
https://doi.org/10.1002/chem.202002767 .
[1]
A. Bonfiglio, K. Magra, C. Cebrian, F. Polo, P.C. Gros, P. Mercandelli, M. Mauro, Red-emitting neutral rhenium(I) complexes bearing a pyridyl pyridoannelated N-heterocyclic carbene, Dalton Transactions 49 (2020) 3102–3111.
https://doi.org/10.1039/c9dt04890a .
[1]
M. Bouche, B. Vincent, T. Achard, S. Bellemin-Laponnaz, N-Heterocyclic Carbene Platinum(IV) as Metallodrug Candidates: Synthesis and(195)Pt NMR Chemical Shift Trend, Molecules 25 (2020) 3148.
https://doi.org/10.3390/molecules25143148 .
[1]
E. Charignon, M. Bouche, C. Clave-Darcissac, G. Dahm, G. Ichim, A. Clotagatide, H.C. Mertani, P. Telouk, J. Caramel, J.-J. Diaz, S. Bellemin-Laponnaz, P. Bouvet, C. Billotey, In Cellulo Evaluation of the Therapeutic Potential of NHC Platinum Compounds in Metastatic Cutaneous Melanoma, International Journal of Molecular Sciences 21 (2020) 7626.
https://doi.org/10.3390/ijms21217826 .
[1]
W. Chen, J. Egly, A. Pobtador-Bahamonde I., A. Maisse-Francois, S. Bellemin-Laponnaz, T. Achard, Synthesis, characterization, catalytic and biological application of half-sandwich ruthenium complexes bearing hemilabile (kappa 2-C,S)-thioether-functionalised NHC ligands, Dalton Transactions 49 (2020) 3243–3252.
https://doi.org/10.1039/c9dt04825a .
[1]
Y. Geiger, T. Achard, A. Maisse-Francois, S. Bellemin-Laponnaz, Observation of hyperpositive non-linear effect in catalytic asymmetric organozinc additions to aldehydes, Chirality (2020) 1–7.
https://doi.org/10.1002/chir.23271 .
[1]
Y. Geiger, T. Achard, A. Maisse-Francois, S. Bellemin-Laponnaz, Hyperpositive non-linear effects: enantiodivergence and modelling, Chemical Science 11 (2020) 12453–12463.
https://doi.org/10.1039/d0sc04724d .
[1]
K.S. Kumar, I. Salitros, B. Heinrich, S. Moldovan, M. Mauro, M. Ruben, Spin-crossover in iron(II)-phenylene ethynylene-2,6-di(pyrazol-1-yl) pyridine hybrids: toward switchable molecular wire-like architectures, Journal of Physics-Condensed Matter 32 (2020) 204002.
https://doi.org/10.1088/1361-648X/ab6cc2 .
[1]
M. Marinova, A. Bonnefont, T. Achard, A. Maisse-Francois, S. Bellemin-Laponnaz, Chiral stimuli-responsive metallo-supramolecular assembly induced by Cu-II/Cu(I)redox change, Chemical Communications 56 (2020) 8703–8706.
https://doi.org/10.1039/d0cc01716g .
[1]
M. Paolino, A. Reale, G. Magrini, V. Razzano, G. Giuliani, A. Donati, G. Giorgi, F. Samperi, M. Canetti, M. Mauro, F. Villafiorita-Monteleone, E. Fois, C. Botta, A. Cappelli, UV-light-induced polymerization in the amorphous solid-state of a spontaneously non-polymerizing 3-phenylbenzofulvene monomer, European Polymer Journal 137 (2020) 109923.
https://doi.org/10.1016/j.eurpolymj.2020.109923 .
[1]
C. Romain, S. Bellemin-Laponnaz, S. Dagorne, Recent progress on NHC-stabilized early transition metal (group 3-7) complexes: Synthesis and applications, Coordination Chemistry Reviews 422 (2020) 213411.
https://doi.org/10.1016/j.ccr.2020.213411 .
[1]
R. Verron, T. Achard, C. Seguin, S. Fournel, S. Bellemin-Laponnaz, Synthesis and Characterization of N-Heterocyclic Carbene Dithiocarbamate Platinum Complexes with Antitumoral Activity, European Journal of Inorganic Chemistry 2020 (2020) 2552–2557.
https://doi.org/10.1002/ejic.202000329 .
Metals and their functions – Events and news