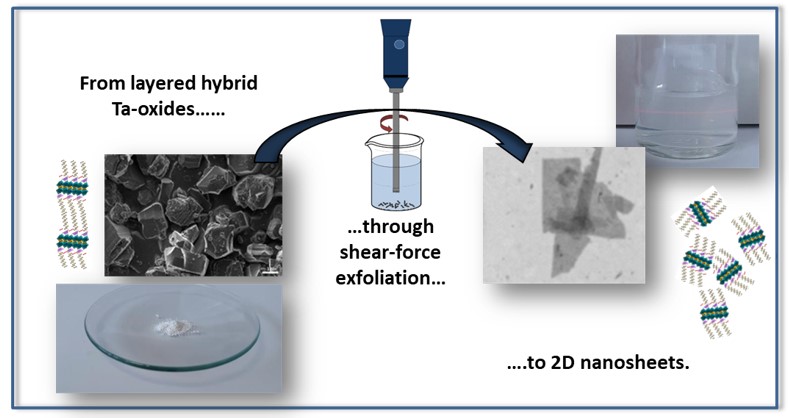
In view of the better understanding we have acquired of the insertion chemistry in layered oxides, we are currently seeking to extend our research to the exfoliation of these objects. The first step is to try to play on the functionalization of the starting lamellar oxide (bulk) in order to favour the exfoliation in liquid phase (exfoliation time, yield, quality of the sheets), and to ensure the stability of the obtained sheets in suspension.
A review of the literature on liquid phase exfoliation reveals two main methods.
The most common and referenced method, which can be used for oxides with negatively charged layers, consists in the exchange of cations in the interlamellar space using a bulky cation.[1-4] It is generally carried out in an aqueous medium for a relatively long reaction time (of the order of about ten days). To our knowledge, it is only used on the protonated phases of lamellar oxides and thus leads to “naked” layers.
The second method of exfoliation in liquid phase commonly used in the literature is of mechanical nature. It relies on the application of shear forces on the sheets of the starting material to separate them.[5,6] It is mainly used for materials with neutral sheets, interacting by Van der Waals forces (graphene, TMD…). In our case, the functionalization of the starting lamellar oxide (by alcohols for example) allows to decrease the density of surface charges, and to reduce the cohesive forces between sheets.
We have therefore started, with very encouraging results, to use shear forces to exfoliate lamellar oxides, hoping to retain the initial functionalization. The essential parameters to control in this method are the exfoliation solvent, the shear rate (speed) and the reaction time. We varied these parameters to estimate the best conditions to obtain nanosheets in solution. We have shown that this synthesis route can indeed produce oxide nanosheets. We are currently working on the optimization of the synthesis conditions, and the fine characterization of the obtained nanosheets (geometrical and chemical characteristics as well as behavior in suspension).
By static and dynamic light scattering at multiple angles, we access the hydrodynamic diameter of the objects and their diffusion coefficient. This technique also attests the stability in time of the functionalized nano-objects (collaboration François Schosseler, ICS, Strasbourg).[7]
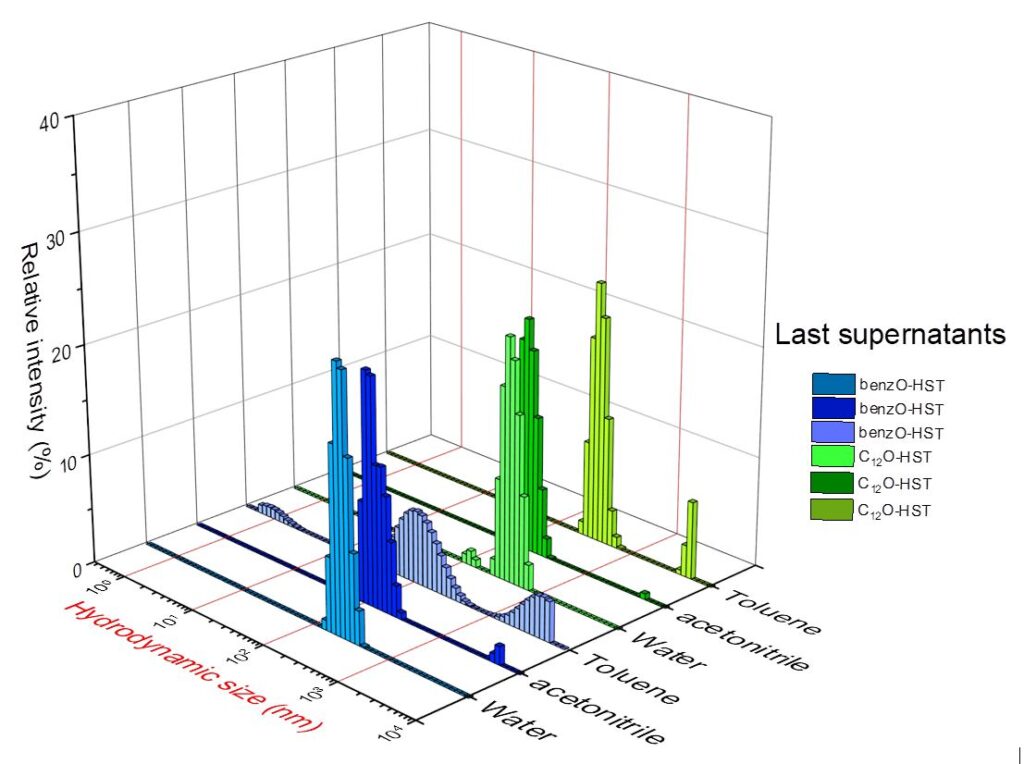
Example of characterization by DLS, influence of the exfoliation solvent and of functionalization of the starting oxide (correlogramms are not shown here but are compulsory to attest the validity of the DLS measure).
Transmission and scanning tunneling electron microscopies confirm that nanosheets are obtained (as well as the presence of non-exfoliated material). The associated electron diffraction allows to have structural information on the hybrid sheets and to compare them with the solid starting material.
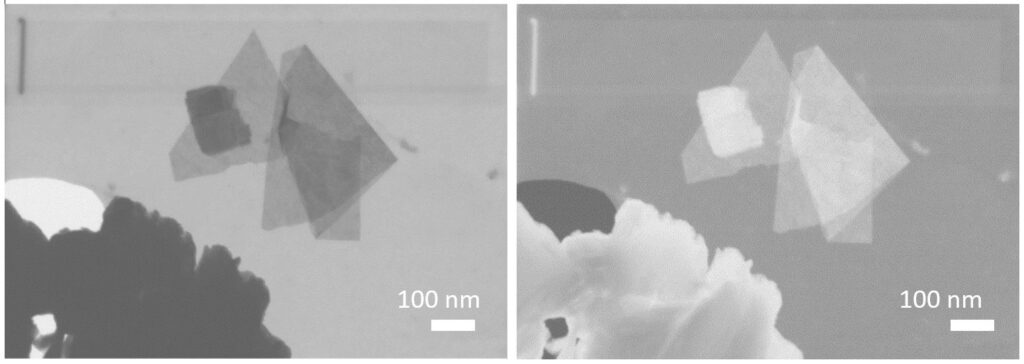
STEM images in dark field (left) and bright field (right)of nanosheets obtained from exfoliation of C12O-HST.
[1] T. Sasaki, M. Watanabe, J. Am. Chem. Soc. 1998, 120, 4682–4689.
[2] H. Yuan, D. Dubbink, R. Besselink, J. E. ten Elshof, Angew. Chem. Int. Ed. 2015, 54, 9239–9243.
[3] R. Uppuluri, A. S. Gupta, A. S. Rosas, T. E. Mallouk, Chem. Soc. Rev. 2018, 47, 2401–2430.
[4] F. Geng, R. Ma, Y. Ebina, Y. Yamauchi, N. Miyamoto, T. Sasaki, J. Am. Chem. Soc. 2014, 136, 5491–5500.
[5] J. N. Coleman, M. Lotya, A. O’Neill, S. D. Bergin, P. J. King, U. Khan, K. Young, A. Gaucher, S. De, R. J. Smith, I. V. Shvets, S. K. Arora, G. Stanton, H.-Y. Kim, K. Lee, G. T. Kim, G. S. Duesberg, T. Hallam, J. J. Boland, J. J. Wang, J. F. Donegan, J. C. Grunlan, G. Moriarty, A. Shmeliov, R. J. Nicholls, J. M. Perkins, E. M. Grieveson, K. Theuwissen, D. W. McComb, P. D. Nellist, V. Nicolosi, Science 2011, 331, 568–571.
[6] K. R. Paton, E. Varrla, C. Backes, R. J. Smith, U. Khan, A. O’Neill, C. Boland, M. Lotya, O. M. Istrate, P. King, T. Higgins, S. Barwich, P. May, P. Puczkarski, I. Ahmed, M. Moebius, H. Pettersson, E. Long, J. Coelho, S. E. O’Brien, E. K. McGuire, B. M. Sanchez, G. S. Duesberg, N. McEvoy, T. J. Pennycook, C. Downing, A. Crossley, V. Nicolosi, J. N. Coleman, Nature Materials 2014, 13, 624–630.
[7] F. Payet, C. Bouillet, F. Leroux, C. Leuvrey, P. Rabu, F. Schosseler, C. Taviot-Guého, G. Rogez, Journal of Colloid and Interface Science 2022, 607, 621–632.
