Low dimensional magnetic systems
We develop different techniques allowing a quantitative analysis of magnetic interactions in low dimensional compounds (0D, 1D, 2D) from the parameterization of experimental magnetic measurements. 1-4] We have particularly studied transition metal hydroxynitrates which have proven to be very good model compounds to understand the effects of dimensionality (structural and spin) and anisotropy on the behavior of 1D or 2D magnetic systems with competing interactions. This type of analysis of the links between structure and property extends to various coordination networks (from isolated complexes to MOFs) synthesized in our team or of interest to colleagues from other laboratories.[5-8] Some literal expressions of the magnetic susceptibility for complex chains have been established and are used in the literature. Our approach is sometimes completed by DFT calculations of the electronic structure of the solid.
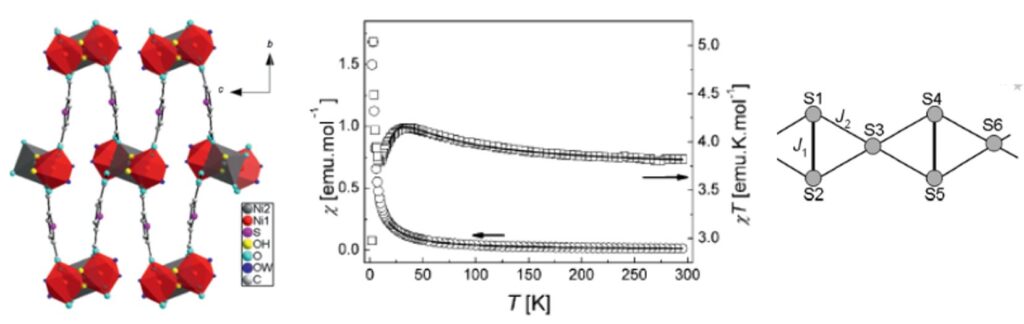
View of the structure of [Ni3(µ3-OH)2((k1-k1)-(k1-k1)-µ4-TDC)2(H2O)4]n along a (left) and thermal variation of the magnetic susceptibility (the solid line corresponds to the best parameterization) (middle). Scheme of the interactions considered in the chain model used for the parameterization (right).[9]
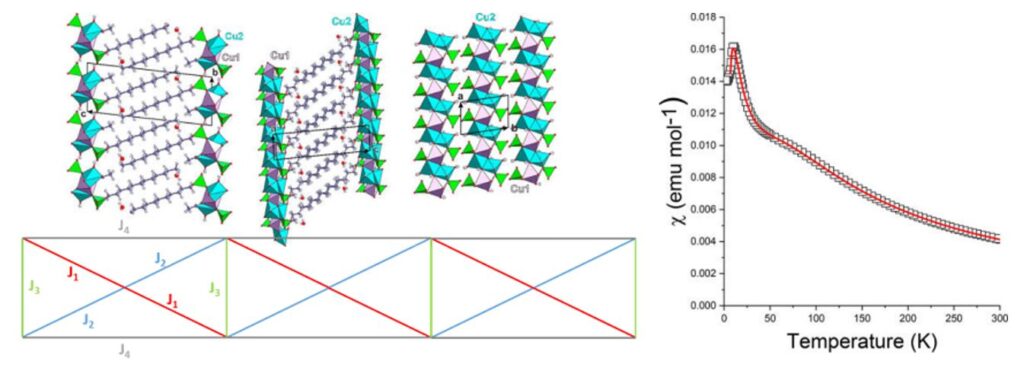
Views of the structure of [Cu3(C12H25SO4)2(CH3COO)2(OH)2(H2O)2]n (top-left) and scheme of the interactions considered in the chain model used for parameterization (bottom-left). Thermal variation of the magnetic susceptibility (the solid line corresponds to the best parameterization) (right).[10]
Isolated molecular systems, magnetic couplings and slow relaxation of magnetization
Part of our activities consists in studying the magnetic properties of discrete molecular systems, to determine the magnetic coupling and anisotropy parameters and the possible slow relaxation of the magnetization. These studies are carried out in collaboration with various colleagues in France and abroad.
As an example, in collaboration with Emilie Delahaye (LCC, Toulouse), we have studied the magnetic properties of a Dy(III) complex of formula [BiCNIm]3[DyCl6] where BiCNIm is the 1,3-bis((cyanomethyl)imidazolium) ligand. The coordination sphere of the Dy(III) ion is formed only by chloride ions. This compound exhibits a slow relaxation of magnetization, which involves Orbach, Raman and QTM mechanisms.[11]
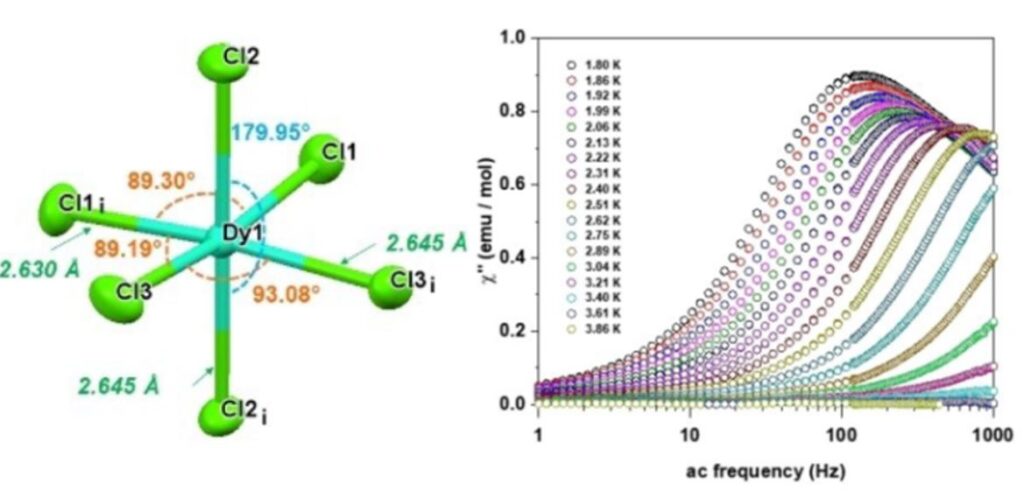
View of the Dy(III) coordination sphere in the compound [BiCNIm]3DyCl6. Out-of-phase phase susceptibility measured under a static field of 500 Oe at different temperatures (right).[11]
In collaboration with V. Chandrasekhar (Kanpur, India), we are interested in the slow relaxation properties of magnetization in 4f, and 3d-4f compounds.[12-14]
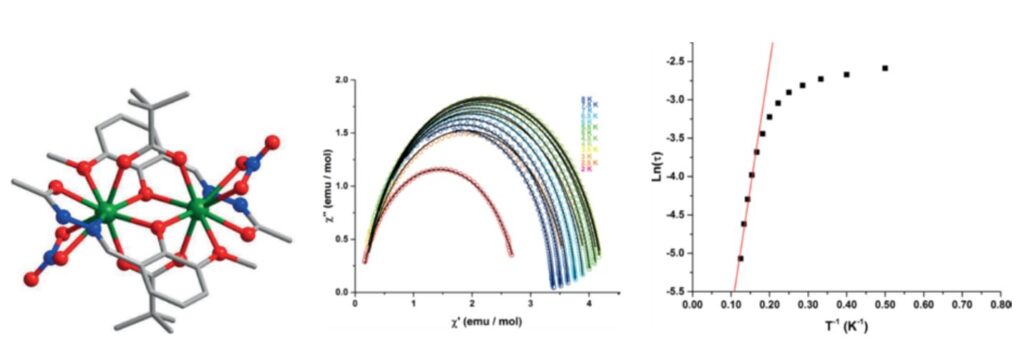
Structural view of the compounds [Ln2(LH)2(μ2-Piv-k2O,O′)2(NO3-k2O,O′)2] (Piv = pivalic acid (trimethylacetic acid), LH2 = 2-hydroxy-3-methoxybenzylidene)acetohydrazide, Ln = Dy3+, Gd3+, Ho3+, Tb3+) (left), Cole-Cole curves for the compound Dy, under zero static field (middle), characteristic magnetization relaxation time (tau), as a function of 1/T. [13]
[1] J.-M. Rueff, N. Masciocchi, P. Rabu, A. Sironi, A. Skoulios, Chem. Eur. J. 2002, 8, 1813–1820.
[2] J. Souletie, P. Rabu, M. Drillon, in Magnetism: Molecules to Materials V (Eds.: J.S. Miller, M. Drillon), Wiley-VCH, Weinheim, 2005.
[3] A. Beghidja, G. Rogez, P. Rabu, R. Welter, M. Drillon, J. Mater. Chem. 2006, 16, 2715–2728.
[4] E. M. Bauer, C. Bellitto, G. Righini, M. Colapietro, G. Portalone, M. Drillon, P. Rabu, Inorg. Chem. 2008, 47, 10945–10952.
[5] C. Livage, N. Guillou, P. Rabu, P. Pattison, J. Marrot, G. Férey, Chem. Commun. 2009, 4551–4553.
[6] A. Mantion, L. Massüger, P. Rabu, C. Palivan, L. B. McCusker, A. Taubert, J. Am. Chem. Soc. 2008, 130, 2517–2526.
[7] C. Falaise, C. Volkringer, J.-F. Vigier, A. Beaurain, P. Roussel, P. Rabu, T. Loiseau, J. Am. Chem. Soc. 2013, 135, 15678–15681.
[8] S. Duval, S. Béghin, C. Falaise, X. Trivelli, P. Rabu, T. Loiseau, Inorg. Chem. 2015, 54, 8271–8280.
[9] A. Demessence, A. Mesbah, M. François, G. Rogez, P. Rabu, Eur. J. Inorg. Chem. 2009, 2009, 3713–3720.
[10] Q. Evrard, C. Leuvrey, P. Farger, E. Delahaye, P. Rabu, G. Taupier, K. D. Dorkenoo, J.-M. Rueff, N. Barrier, O. Pérez, G. Rogez, Crystal Growth & Design 2018, 18, 1809–1817.
[11] N. Benamara, M. Diop, C. Leuvrey, M. Lenertz, P. Gilliot, M. Gallart, H. Bolvin, F. Setifi, G. Rogez, P. Rabu, E. Delahaye, Eur. J. Inorg. Chem. 2021, 2021, 2099–2107.
[12] P. Bag, A. Chakraborty, G. Rogez, V. Chandrasekhar, Inorg. Chem. 2014, 53, 6524–6533.
[13] S. Biswas, S. Das, G. Rogez, V. Chandrasekhar, Eur. J. Inorg. Chem. 2016, 3322–3329.
[14] J. Goura, R. Guillaume, E. Rivière, V. Chandrasekhar, Inorg. Chem. 2014, 53, 7815–7823.
