Luminescent liquid materials: from fundamental research to applications
Laurent Douce
Cristaux liquides ioniques luminescents basés sur l’unité Fluorophore-Imidazolium
The scientific interest in ionic materials (ionic liquids) is due to their high chemical modularity. They have unique properties, for example, no vapor pressure, high thermal stability, non-flammability, chemical and radiochemical stability, ionic conductivity and a wide electrochemical window. Moreover, by playing on the nature of the anions, it is possible to fine tune the physical properties of these salts such as viscosity, melting point, polarity… The organic cationic part affects the amphipathic character and the weak bonds/interactions that control the self-organization architectures in the mesomorphic and crystalline state. In recent years, we have developed a synthetic methodology to prepare these luminescent salts in only three steps based on imidazolium at the scale of a hundred grams. This approach allowed us to obtain fluorescent salts in pure liquids, crystal-liquids and crystals with high luminescence quantum yields of the order of 80%.


- del Giudice, N. & al. European Journal of Organic Chemistry 2021. DOI :10.1002/ejoc.202100047 and cover page DOI : 10.1002/ejoc.202100374.
Luminescent ionic materials for biological tools
The organic cation backbone affects the amphipathic character (balance between two antagonistic parts: rigid aromatic part / flexible alkyl chains) which controls supramolecular architectures. These unique properties have led to applications for example in biology (targeting and transport of drugs and genetic materials (RNA interference transfections)).
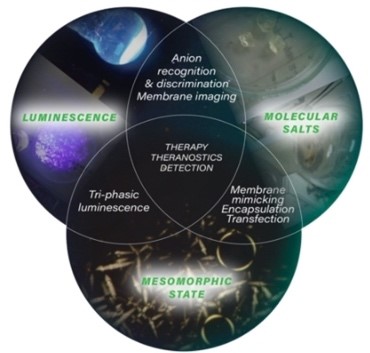
Luminescent ionic materials towards applications for Neutron/Gamma detection
These scintillating saline materials are able to detect neutrons whatever their energies by discriminating them from Gamma rays. We are leading the valorisation (prematuration-CNRS Luxcifer) of these materials in collaboration with industrials for the detection of radioactive radiation and thus replace helium neutron detectors(3). A prototype was successfully tested in January 2021 at the Fessenheim nuclear power plant to detect a boron oxide plug in its primary circuit (co-developed with IPHC/CENBG and the companies Carmelec and EdF).

The second prematuration Noctiferium in collaboration with the Néel Institute, allows to acquire skills in crystallogenesis and to shape these materials in order to grow centimetric single crystals to develop devices of type radiameter and Neutron dosimeter. An important facet of this research work will be to shape these materials into optical quality single crystals, to acquire and develop knowledge in crystallogenesis of organic materials. For this, we have built two devices:
1) the crystallization of the dissolved ionic compounds is caused by the lowering of the temperature of the whole solution. The rate of temperature lowering must thus compensate as well as possible the depletion of the solution in ionic compound. This reactor is in function and allows us to obtain centimetric single crystals of our luminescent salts of centimetric sizes.
2) In this second method, crystallization is achieved by circulating the solution between a saturation reactor (usually hotter) and a growth reactor (usually colder). This allows the solution to be kept out of equilibrium in the growth reactor in a perfectly stationary manner and thus ensures perfectly defined stable crystallization conditions.


