Layered hydroxides : model magnetic hybrid compounds
Layered Simple Hydroxides have a general formula M2(OH)3X (MII = Co, Cu, Ni, Mn and X– = NO3–, Cl–, carboxylate, sulfonate, sulfate or phosphonate).
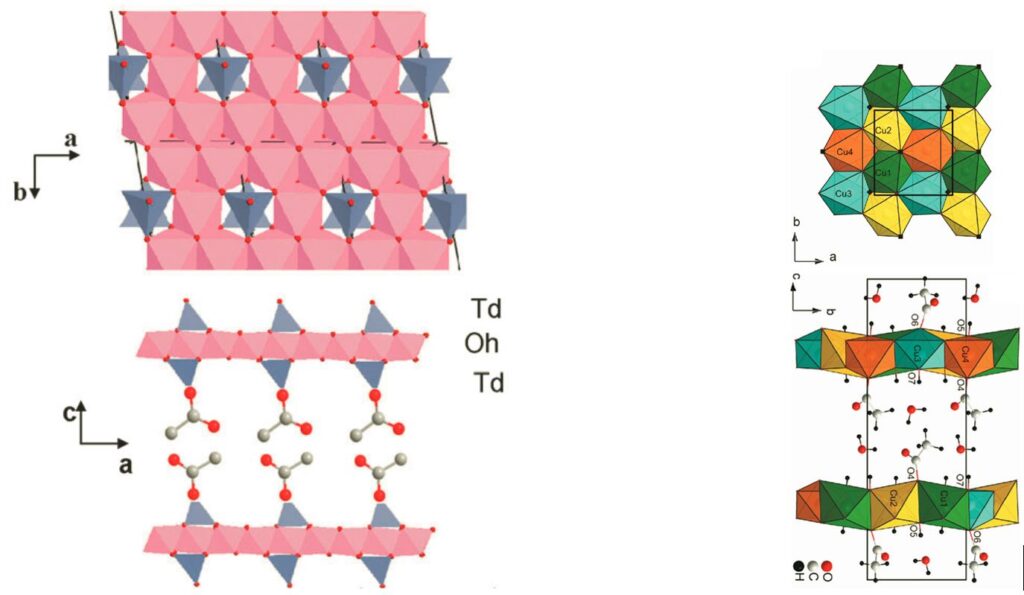
A remarkable characteristic of these systems is that the anion present in the interlamellar space is generally coordinated to the metals of the inorganic layer. This anion, an acetate ligand for example, can be substituted by a large number of molecules possessing a “grafting” function, sulfonate or carboxylate in particular, via an anionic exchange reaction. The species thus intercalated can serve as a pillar or even as connector between the inorganic layers. In such systems, the covalency of the bond between the intercalated species and the inorganic sublayer favors the possible interaction between properties.[5]
First, we investigated the interaction mechanisms and structural factors influencing the magnetic properties of LSH-based hybrids. Then, increasingly complex anions were immobilized and grafted within the interlayer space to study the possibility of new functionalities. These systems are good models to understand the correlations between structure and physical properties provided by molecular entities grafted onto hydroxide sheets by coordination to the metal.
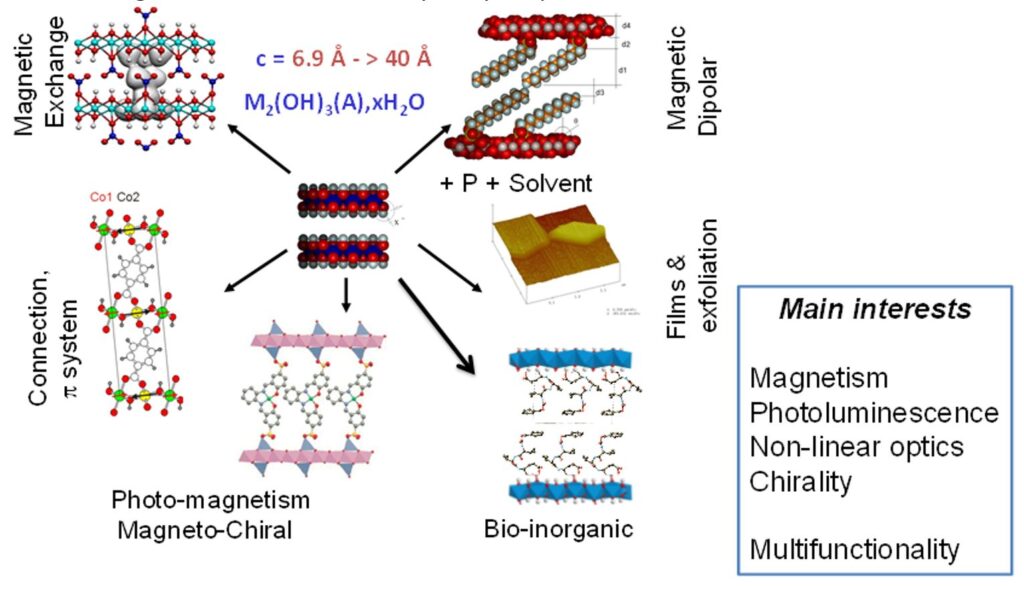
We have especially highlighted the relationships between structure and properties, the different interaction mechanisms (exchange, dipolar, spin polarization) involved in lamellar systems, the influence of the inter-layer distance and the possibility to obtain flexible and adaptable magnetic layered structures.[6-12]
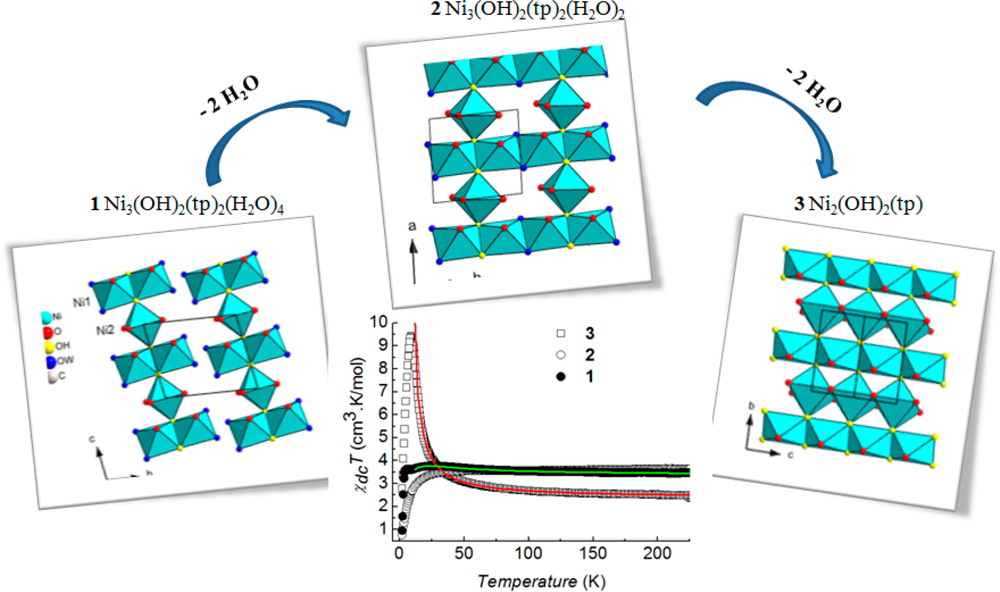
Toward engineering complex multifunctional hybrid materials
The most recent works have focused on the development of anion exchange as a synthetic route. This route is relatively versatile and we have been able to obtain multifunctional compounds by playing with magnetic, fluorescent, chiral or bioinspired organic or organometallic anions. The coupling between properties is at the heart of our current concerns.
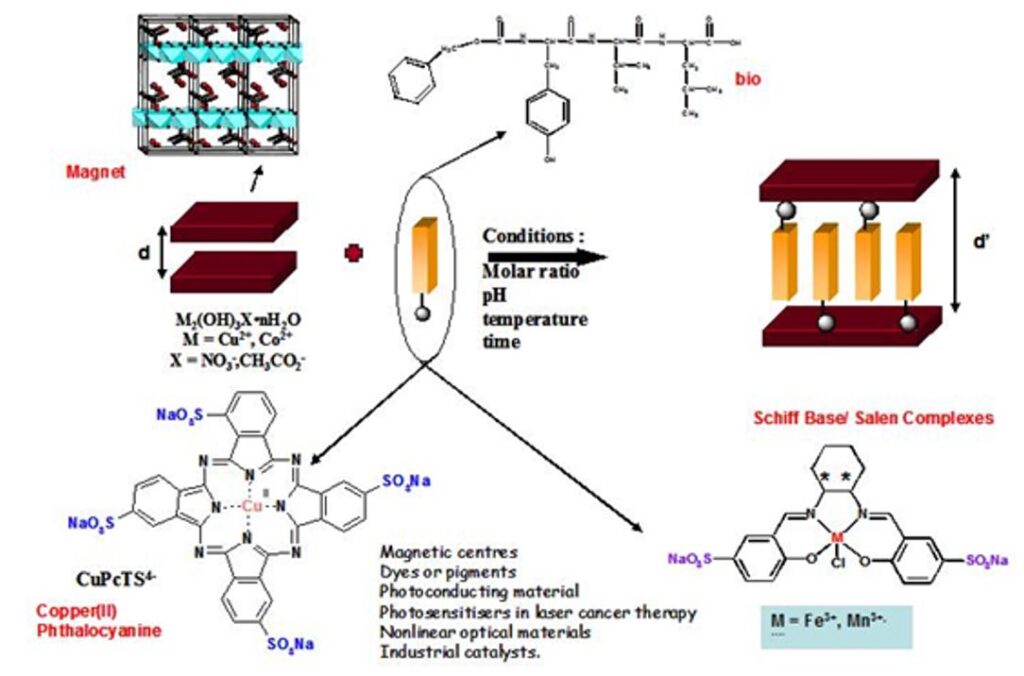
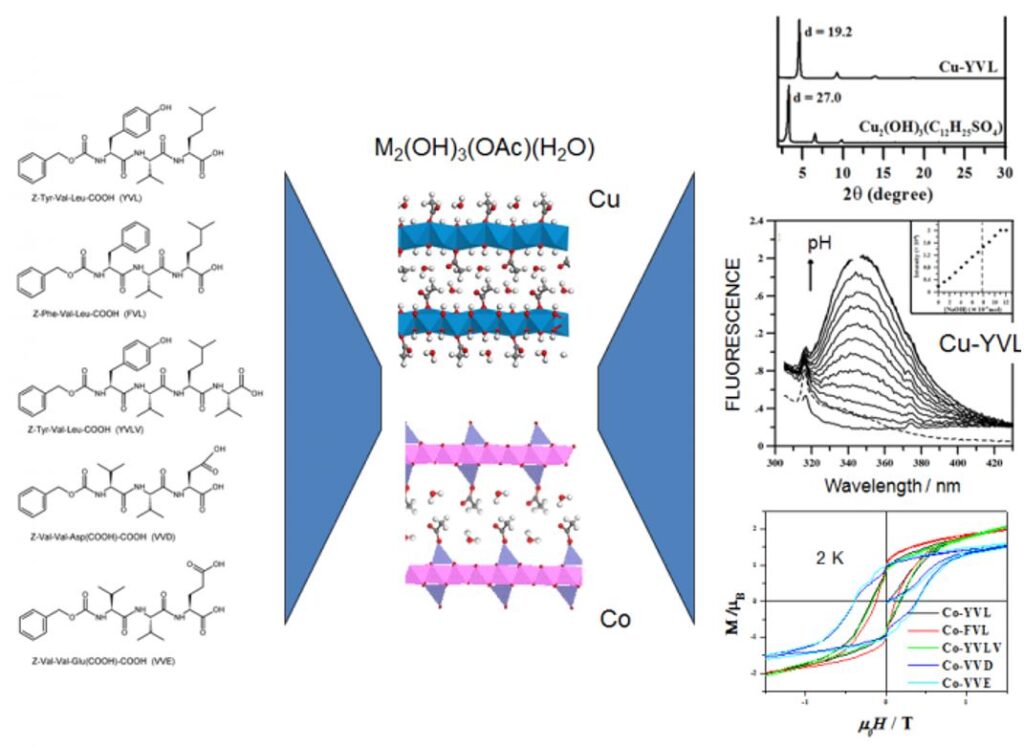
Some results concern the coupling between magnetism and optical properties. In the case of hydroxides functionalized with oligopeptides, we have been able to study the effect of the presence of redox and fluorescent groups as well as of the nature of the pendant chains on the overall properties of the final hybrid material. Some rules can be proposed for the synergy to take place between magnetism and optical properties (grafting mode, luminescence yield, λabs/em, excited state lifetime).
This approach of bi-functional material is interesting because the grafting of photo-active molecules within a crystallized structure allows to obtain a network of organized molecules which can create a cooperative effect. The complexes or organic molecules can play a structuring role or the on of an electronic coupler (conjugated systems) and the existence of an iono-covalent bond between the sub-networks favors a synergy between the properties of each entity. Similarly, we are interested in the structuring of functional solids by molecules with self-assembly properties. The aim is to study the effect of a rearrangement of the interlayer network on the properties as well as the effect of confinement on the self-assembly. The recent results obtained, including in other systems than hydroxides, show that our approach is relevant to obtain complex multifunctional materials.[5,13-18]
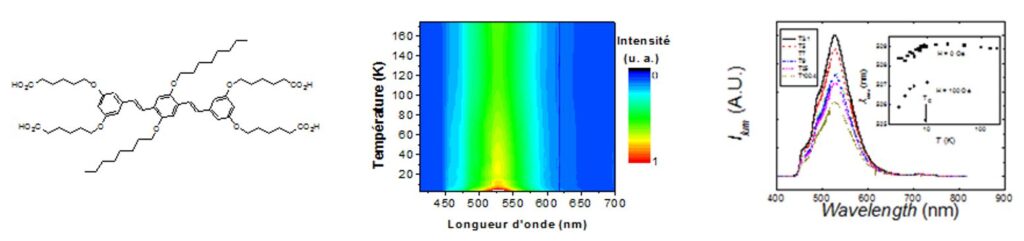
OPV-tetracarboxylate molecule inserted between the layers of a Ni(II) hydroxyde and temperature variation of the ligand luminescence in the hybrid compound.[23]
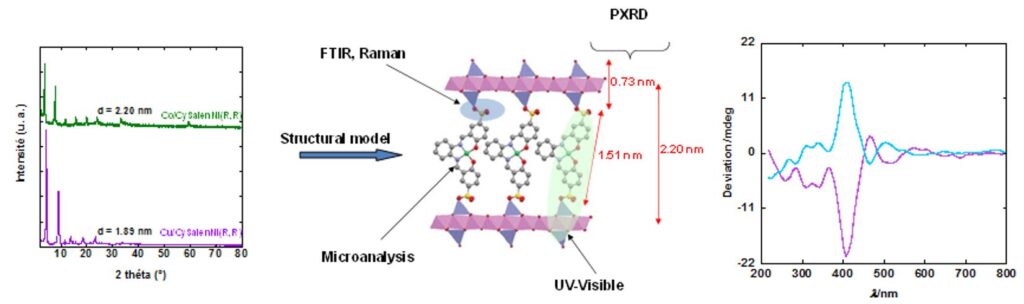
X-ray diffraction pattern of Ni((R,R)CySalenSO3)@Cu (green) et Ni((R,R)CySalenSO3)@Co (purple) (Cu Kα1 = 0.1540598 nm) and structural model. Optical circular dichroïsm spectra of the hybrid compounds, indicating the chirality of the complex is preserved after grafting within the layered structure. Hence, the insertion of chiral nickel complexes allows the convey the hybrid materials a chiral character.[22]
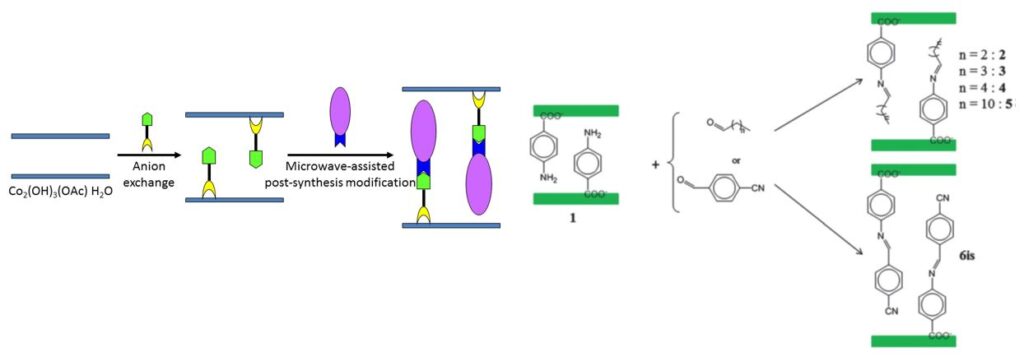
One of the last important results that we obtained in the field of multifunctional lamellar simple hydroxides concerns the synthesis, characterization and study of a cobalt hydroxide based compound, functionalized by a fluorene phosphonate ligand showing an important magneto-electric effect. This work was carried out within the framework of the ANR ” HYMN ” (HYbrid Multiferroic Nanomaterials) (partners : J.-M. Rueff, CRISMAT, Caen and P.-A. Jaffrès, CEMCA, Brest).
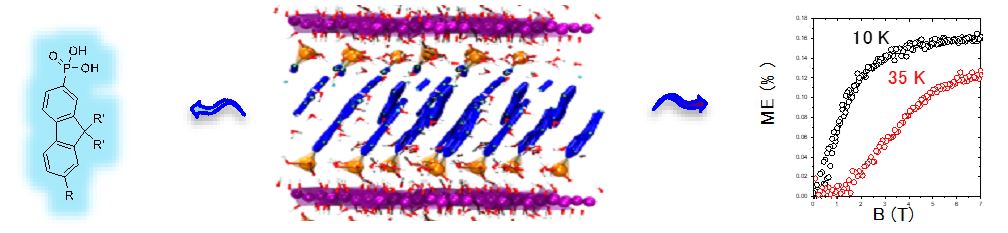
Cobalt hydroxide de cobalt functionalized by fluorene-phosphonate ligands (collaboration P.-A. Jaffrès, CEMCA), structural modelling (collaboration G. Ori, IPCMS), and magneto-electric properties (collaboration A. Pautrat, CRISMAT).[25]
[1] M. Louër, D. Louër, D. Grandjean, Acta Cryst. Sect. B 1973, 29, 1696–1703.
[2] V. Laget, C. Hornick, P. Rabu, M. Drillon, J. Mater. Chem. 1999, 9, 169–174.
[3] L. Poul, N. Jouini, F. Fiévet, Chem. Mater. 2000, 12, 3123–3132.
[4] S. Švarcová, M. Klementová, P. Bezdička, W. Łasocha, M. Dušek, D. Hradil, Cryst. Res. Technol. 2011, 46, 1051–1057.
[5] G. Rogez, C. Massobrio, P. Rabu, M. Drillon, Chem. Soc. Rev. 2011, 40, 1031–1058.
[6] V. Laget, C. Hornick, P. Rabu, M. Drillon, R. Ziessel, Coordination Chemistry Reviews 1998, 178–180, 1533–1553.
[7] Z.-L. Huang, M. Drillon, N. Masciocchi, A. Sironi, J.-T. Zhao, P. Rabu, P. Panissod, Chem. Mater. 2000, 12, 2805–2812.
[8] R. Feyerherm, A. Loose, P. Rabu, M. Drillon, Solid State Sciences 2003, 5, 321–326.
[9] P. Rabu, M. Drillon, in Functional Hybrid Materials (Eds.: P. Gómez-Romero, C. Sanchez), Wiley-VCH, Weinheim, 2004, pp. 270–313.
[10] E. Ruiz, M. Llunell, J. Cano, P. Rabu, M. Drillon, C. Massobrio, J. Phys. Chem. B 2006, 110, 115–118.
[11] A. Demessence, G. Rogez, P. Rabu, Chem. Mater. 2006, 18, 3005–3015.
[12] A. Mesbah, P. Rabu, R. Sibille, S. Lebègue, T. Mazet, B. Malaman, M. François, Inorg. Chem. 2014, 53, 872–881.
[13] É. Delahaye, M. Diop, R. Welter, M. Boero, C. Massobrio, P. Rabu, G. Rogez, Eur. J. Inorg. Chem. 2010, 2010, 4450–4461.
[14] A. Demessence, A. Yassar, G. Rogez, L. Miozzo, S. De Brion, P. Rabu, J. Mater. Chem. 2010, 20, 9401–9414.
[15] E. Delahaye, Z. Xie, A. Schaefer, L. Douce, G. Rogez, P. Rabu, C. Günter, J. S. Gutmann, A. Taubert, Dalton Trans. 2011, 40, 9977–9988.
[16] E. Delahaye, S. Eyele-Mezui, M. Diop, C. Leuvrey, D. Foix, D. Gonbeau, P. Rabu, G. Rogez, European Journal of Inorganic Chemistry 2012, 2731–2740.
[17] S. Si, A. Taubert, A. Mantion, G. Rogez, P. Rabu, Chem. Sci. 2012, 3, 1945–1957.
[18] O. Palamarciuc, E. Delahaye, P. Rabu, G. Rogez, New J. Chem. 2014, 38, 2016–2023.
[19] R. Bourzami, S. Eyele-Mezui, E. Delahaye, M. Drillon, P. Rabu, N. Parizel, S. Choua, P. Turek, G. Rogez, Inorg. Chem. 2014, 53, 1184–1194.
[20] S. Eyele-Mezui, P. Vialat, C. Higy, R. Bourzami, C. Leuvrey, N. Parizel, P. Turek, P. Rabu, G. Rogez, C. Mousty, Journal of Physical Chemistry C 2015, 119, 13335–13342.
[21] S. Eyele-Mezui, E. Delahaye, G. Rogez, P. Rabu, European Journal of Inorganic Chemistry 2012, 5225–5238.
[22] É. Delahaye, S. Eyele-Mezui, M. Diop, C. Leuvrey, P. Rabu, G. Rogez, Dalton Trans. 2010, 39, 10577–10580.
[23] J.-M. Rueff, J.-F. Nierengarten, P. Gilliot, A. Demessence, O. Cregut, M. Drillon, P. Rabu, Chem. Mater. 2004, 16, 2933–2937.
[24] Y. Wang, E. Delahaye, C. Leuvrey, F. Leroux, P. Rabu, G. Rogez, Inorg. Chem. 2016, 55, 9790–9797.
[25] Q. Evrard, Z. Chaker, M. Roger, C. M. Sevrain, E. Delahaye, M. Gallart, P. Gilliot, C. Leuvrey, J.-M. Rueff, P. Rabu, C. Massobrio, M. Boero, A. Pautrat, P.-A. Jaffrès, G. Ori, G. Rogez, Adv. Funct. Mater. 2017, 1703576.
