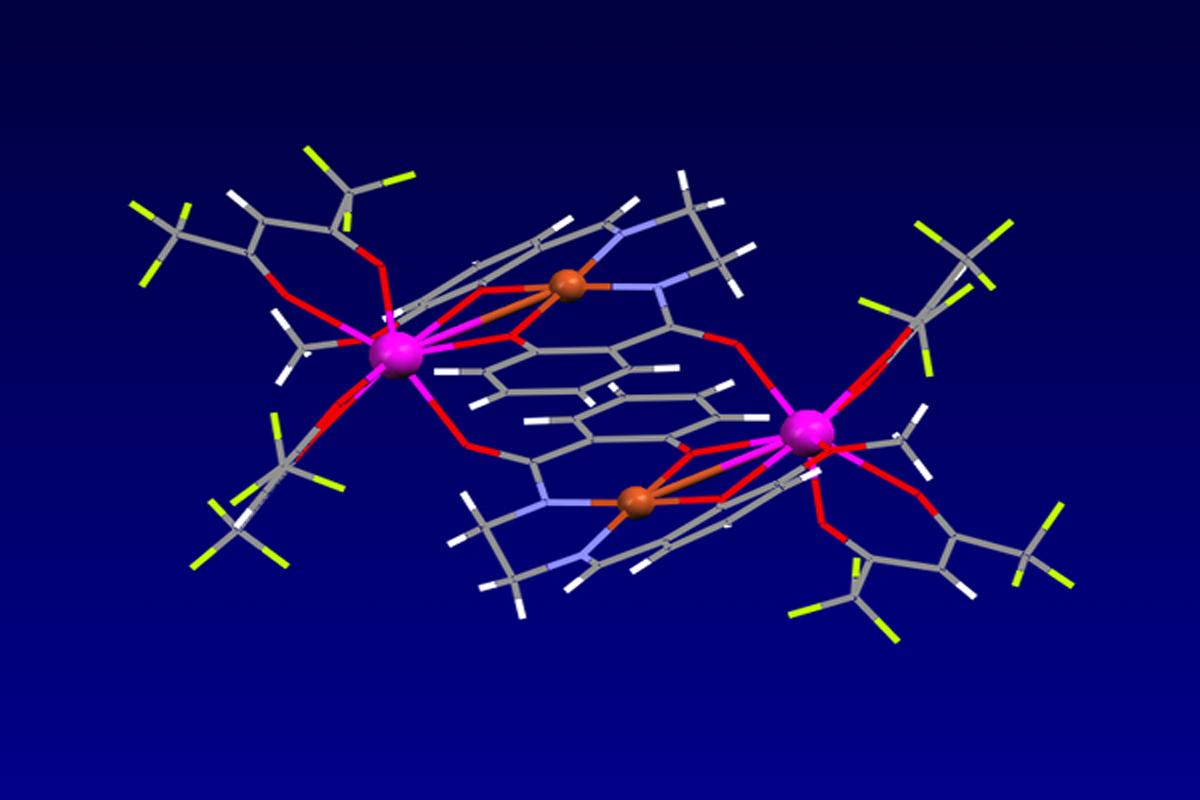
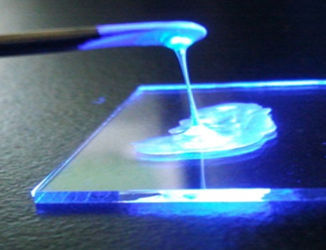
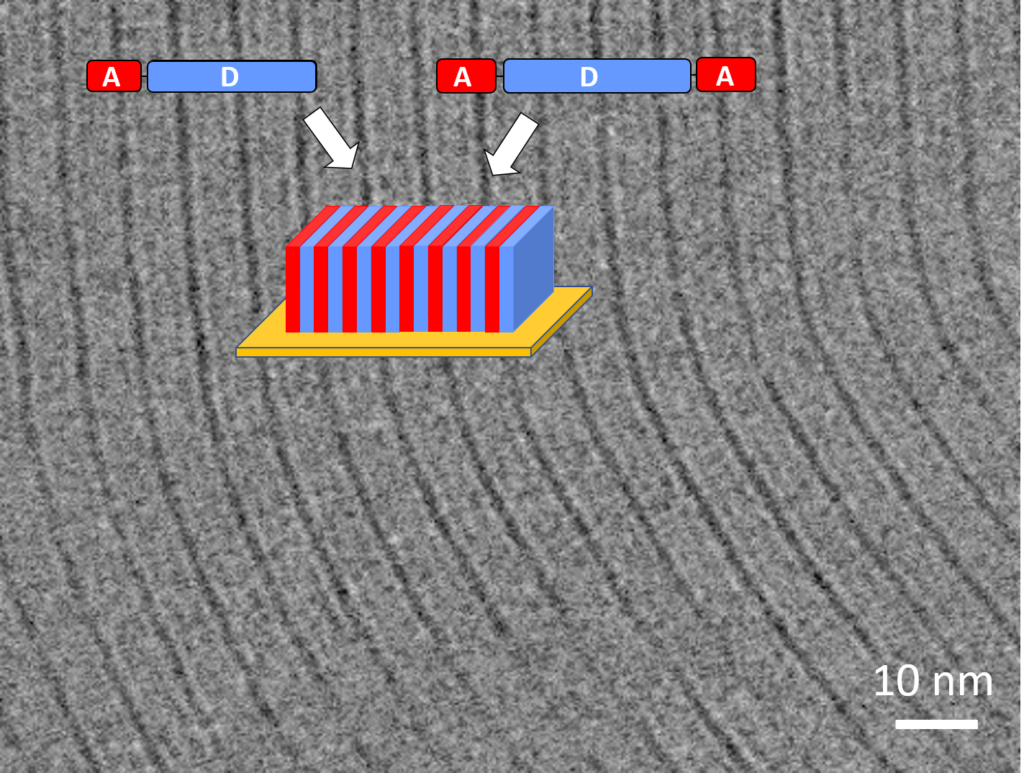
L’activité de l’équipe porte sur le développement des systèmes moléculaires présentant notamment des propriétés de fluorescence ou de transport de charge pour des applications variées telles que les diodes laser, le photovoltaïque, les détecteurs de neutrons… Une attention toute particulière est portée sur le contrôle de l’organisation de ces systèmes moléculaires qui peuvent être liquides, mésomorphes ou monocristallins. Outre la conception et la synthèse de ces systèmes moléculaires, l’équipe s’intéresse également à l’étude des propriétés photophysiques.
Les activités
Le personnel

Doctorant, Matériaux Organiques (DMO)romain.berthiot@ipcms.unistra.fr
Tél: +33(0)3 88 10 71 57Bureau: 2036
Doctorant, Matériaux Organiques (DMO)lilian.colin@ipcms.unistra.fr
Tél: +33(0)3 88 10 71 62Bureau: 2041

Directeur de Recherche, Matériaux Organiques (DMO)anthony.daleo@ipcms.unistra.fr
Voir la page personnelleTél: +33(0)3 88 10 71 47Bureau: 2033

Doctorant, Matériaux Organiques (DMO)nicolas.delgiudice@ipcms.unistra.fr
Voir la page personnelleTél: +33(0)3 88 10 71 57Bureau: 2036

Professeur, Matériaux Organiques (DMO)Laurent.Douce@ipcms.unistra.fr
Voir la page personnelleTél: +33(0)3 88 10 71 07Bureau: 2028
Doctorante, Matériaux Organiques (DMO)agathe.hoppenot@ipcms.unistra.fr
Tél: +33(0)3 88 10 70 91Bureau: 1030

Doctorante, Matériaux Organiques (DMO)justine.kapp@ipcms.unistra.fr
Tél: +33(0)3 88 10 71 57Bureau: 2036

Maîtresse de conférences, Matériaux Organiques (DMO)Isabelle.Kraus@ipcms.unistra.fr
Tél: +33(0)3 88 10 71 46Bureau: 2025

Chargé de Recherche, Matériaux Organiques (DMO)Stephane.Mery@ipcms.unistra.fr
Voir la page personnelleTél: +33(0)3 88 10 71 45Bureau: 2044

Doctorant, Matériaux Organiques (DMO)guillaume.michaluszko@ipcms.unistra.fr
Tél: +33(0)3 88 10 70 91Bureau: 1030

Doctorante, Matériaux Organiques (DMO)yamini.sharma@ipcms.unistra.fr
Tél: +33(0)3 88 10 70 91Bureau: 1030

Nous sommes membres du consortium de laboratoires strasbourgeois sur l’électronique organique (STELORG)!
Les publications récentes
1839302
VYTETDZF
1
surface-science-reports
50
creator
desc
year
1529
https://www.ipcms.fr/plugins/zotpress/
%7B%22status%22%3A%22success%22%2C%22updateneeded%22%3Afalse%2C%22instance%22%3Afalse%2C%22meta%22%3A%7B%22request_last%22%3A50%2C%22request_next%22%3A50%2C%22used_cache%22%3Atrue%7D%2C%22data%22%3A%5B%7B%22key%22%3A%22WLBDDA62%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Zhu%20et%20al.%22%2C%22parsedDate%22%3A%222021%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EX.%20Zhu%2C%20C.%20Hessin%2C%20A.%20Salame%2C%20L.%20Sosa-Vargas%2C%20D.%20Kreher%2C%20C.%20Adachi%2C%20A.%20Proust%2C%20P.%20Mialane%2C%20J.%20Marrot%2C%20A.%20Bouchet%2C%20M.%20Sliwa%2C%20S.%20M%26%23xE9%3Bry%2C%20B.%20Heinrich%2C%20F.%20Mathevet%2C%20G.%20Izzet%2C%20Photoactive%20Organic%5C%2FInorganic%20Hybrid%20Materials%20with%20Nanosegregated%20Donor-Acceptor%20Arrays%2C%20Angewandte%20Chemie-International%20Edition%2060%20%282021%29%208419%26%23x2013%3B8424.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fanie.202014319%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fanie.202014319%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Photoactive%20Organic%5C%2FInorganic%20Hybrid%20Materials%20with%20Nanosegregated%20Donor-Acceptor%20Arrays%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Xiaolei%22%2C%22lastName%22%3A%22Zhu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Cheriehan%22%2C%22lastName%22%3A%22Hessin%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Aude%22%2C%22lastName%22%3A%22Salame%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Lydia%22%2C%22lastName%22%3A%22Sosa-Vargas%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22David%22%2C%22lastName%22%3A%22Kreher%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Chihaya%22%2C%22lastName%22%3A%22Adachi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Anna%22%2C%22lastName%22%3A%22Proust%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Pierre%22%2C%22lastName%22%3A%22Mialane%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jerome%22%2C%22lastName%22%3A%22Marrot%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Aude%22%2C%22lastName%22%3A%22Bouchet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Michel%22%2C%22lastName%22%3A%22Sliwa%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22St%5Cu00e9phane%22%2C%22lastName%22%3A%22M%5Cu00e9ry%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Benoit%22%2C%22lastName%22%3A%22Heinrich%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Fabrice%22%2C%22lastName%22%3A%22Mathevet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Guillaume%22%2C%22lastName%22%3A%22Izzet%22%7D%5D%2C%22abstractNote%22%3A%22The%20synthesis%20of%20the%20first%20mesogenic%20donor-acceptor%20polyoxometalate%20%28POM%29-based%20hybrid%20is%20herein%20described.%20The%20structural%20and%20electronic%20properties%20of%20the%20hybrid%20compound%20were%20evaluated%20through%20combination%20of%20small-%20and%20wide-angle%20X-ray%20scattering%2C%20optical%20microscopy%2C%20electrochemistry%20and%20photoluminescence.%20In%20the%20solid%20state%2C%20the%20compound%20behaves%20as%20a%20birefringent%20solid%2C%20displaying%20a%20lamellar%20organization%20in%20which%20double-layers%20of%20POMs%20and%20bis%28thiophene%29thienothiophene%20organic%20donors%20alternate%20regularly.%20Noticeably%2C%20the%20sub-unit%20organizations%20in%20the%20composite%20are%20similar%20to%20that%20observed%20for%20the%20individual%20POM%20and%20organic%20donor%20precursors.%20Photophysical%20studies%20show%20that%20in%20the%20hybrid%2C%20the%20fluorescence%20of%20the%20organic%20donor%20unit%20is%20considerably%20quenched%20both%20in%20solution%20and%20in%20the%20solid%20state%2C%20which%20is%20attributed%20to%20occurrence%20of%20intramolecular%20charge-separated%20state.%22%2C%22date%22%3A%222021%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1002%5C%2Fanie.202014319%22%2C%22ISSN%22%3A%221433-7851%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1002%5C%2Fanie.202014319%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22TK3HH32E%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222021-06-09T13%3A03%3A51Z%22%7D%7D%2C%7B%22key%22%3A%223RGRCN64%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Zaborova%20et%20al.%22%2C%22parsedDate%22%3A%222013%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EE.%20Zaborova%2C%20P.%20Chavez%2C%20R.%20Bechara%2C%20P.%20Leveque%2C%20T.%20Heiser%2C%20S.%20M%26%23xE9%3Bry%2C%20N.%20Leclerc%2C%20Thiazole%20as%20a%20weak%20electron-donor%20unit%20to%20lower%20the%20frontier%20orbital%20energy%20levels%20of%20donor-acceptor%20alternating%20conjugated%20materials%2C%20Chemical%20Communications%2049%20%282013%29%209938%26%23x2013%3B9940.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fc3cc45481a%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fc3cc45481a%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Thiazole%20as%20a%20weak%20electron-donor%20unit%20to%20lower%20the%20frontier%20orbital%20energy%20levels%20of%20donor-acceptor%20alternating%20conjugated%20materials%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22E.%22%2C%22lastName%22%3A%22Zaborova%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22P.%22%2C%22lastName%22%3A%22Chavez%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22R.%22%2C%22lastName%22%3A%22Bechara%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22P.%22%2C%22lastName%22%3A%22Leveque%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22T.%22%2C%22lastName%22%3A%22Heiser%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22St%5Cu00e9phane%22%2C%22lastName%22%3A%22M%5Cu00e9ry%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22N.%22%2C%22lastName%22%3A%22Leclerc%22%7D%5D%2C%22abstractNote%22%3A%22We%20demonstrate%20that%20the%20substitution%20of%20the%20thiophene%20by%20the%20thiazole%20heterocyclic%20compound%20as%20a%20weak%20electron-donor%20unit%2C%20in%20donor-acceptor%20alternating%20conjugated%20materials%2C%20allows%20a%20simultaneous%20downshift%20of%20both%20HOMO%20and%20LUMO%20levels%20while%20keeping%20the%20energy%20bandgap%20almost%20unchanged.%22%2C%22date%22%3A%222013%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1039%5C%2Fc3cc45481a%22%2C%22ISSN%22%3A%221359-7345%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1039%5C%2Fc3cc45481a%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22BMA9GKQT%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222021-06-09T12%3A59%3A05Z%22%7D%7D%2C%7B%22key%22%3A%22DF3S2V6H%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Yu%20et%20al.%22%2C%22parsedDate%22%3A%222017%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EW.%20Yu%2C%20C.A.%20Serra%2C%20I.U.%20Khan%2C%20M.%20Er-Rafik%2C%20M.%20Schmutz%2C%20I.%20Kraus%2C%20S.%20Ding%2C%20L.%20Zhang%2C%20M.%20Bouquey%2C%20R.%20Muller%2C%20Development%20of%20an%20Elongational-Flow%20Microprocess%20for%20the%20Production%20of%20Size-Controlled%20Nanoemulsions%3A%20Application%20to%20the%20Preparation%20of%20Monodispersed%20Polymer%20Nanoparticles%20and%20Composite%20Polymeric%20Microparticles%2C%20Macromolecular%20Reaction%20Engineering%2011%20%282017%29%201600025.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fmren.201600025%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fmren.201600025%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Development%20of%20an%20Elongational-Flow%20Microprocess%20for%20the%20Production%20of%20Size-Controlled%20Nanoemulsions%3A%20Application%20to%20the%20Preparation%20of%20Monodispersed%20Polymer%20Nanoparticles%20and%20Composite%20Polymeric%20Microparticles%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Wei%22%2C%22lastName%22%3A%22Yu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Christophe%20A.%22%2C%22lastName%22%3A%22Serra%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ikram%20U.%22%2C%22lastName%22%3A%22Khan%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Meriem%22%2C%22lastName%22%3A%22Er-Rafik%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Marc%22%2C%22lastName%22%3A%22Schmutz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Isabelle%22%2C%22lastName%22%3A%22Kraus%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Shukai%22%2C%22lastName%22%3A%22Ding%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Lixiong%22%2C%22lastName%22%3A%22Zhang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Michel%22%2C%22lastName%22%3A%22Bouquey%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Rene%22%2C%22lastName%22%3A%22Muller%22%7D%5D%2C%22abstractNote%22%3A%22A%20three-step%20microfluidic%20process%20is%20proposed%20for%20the%20production%20of%20composite%20plain%20and%20Janus%20polymeric%20microparticles%20doped%20with%20polymer%20nanoparticles.%20These%20monodispersed%20microparticles%20are%20prepared%20by%20means%20of%20capillaries-based%20microfluidic%20droplet%20generators%20from%20a%20dispersed%20phase%20obtained%20after%20the%20thermally%20induced%20or%20UV-initiated%20miniemulsion%20polymerization%20of%20size-controlled%20oil-in-water%20monomer-based%20nanoemulsions%20produced%20in%20a%20novel%20elongational-flow%20microemulsifier.%20Nanodroplets%20and%20polymer%20microparticles%20sizes%20are%20conveniently%20varied%20by%20tuning%20the%20different%20process%20parameters%2C%20namely%2C%20the%20reciprocating%20flow%20rate%20through%20the%20emulsifier%20microchannel%20and%20number%20of%20cycles%20for%20the%20former%20and%20flow%20rates%20of%20all%20immiscible%20phases%20for%20the%20latter.%20As%20such%2C%20300%20mu%20m%20plain%20poly%28acrylamide%29%20microparticles%20and%20400%20mu%20m%20poly%28acrylamide%29%5C%2Fdoped%20poly%28acrylamide%29%20Janus%20microparticles%20containing%20230%20nm%20poly%28tri%28propylene%20glycol%29%20diacrylate-co-methyl%20methacrylate%29%20nanoparticles%20embedded%20selectively%20into%20one%20poly%28acrylamide%29%20domain%20are%20successfully%20prepared.%20This%20microfluidic%20process%20represents%20a%20facile%20route%20to%20the%20synthesis%20of%20multiscale%20and%20multidomain%20composite%20polymeric%20microparticles.%22%2C%22date%22%3A%222017%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1002%5C%2Fmren.201600025%22%2C%22ISSN%22%3A%221862-832X%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1002%5C%2Fmren.201600025%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22VRM2E3H6%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222022-01-24T13%3A28%3A57Z%22%7D%7D%2C%7B%22key%22%3A%22DVDNVK4I%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Yu%20et%20al.%22%2C%22parsedDate%22%3A%222019%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EW.%20Yu%2C%20N.%20Visaveliya%2C%20C.A.%20Serra%2C%20J.M.%20K%26%23xF6%3Bhler%2C%20S.%20Ding%2C%20M.%20Bouquey%2C%20R.%20Muller%2C%20M.%20Schmutz%2C%20I.%20Kraus%2C%20Preparation%20and%20Deep%20Characterization%20of%20Composite%5C%2FHybrid%20Multi-Scale%20and%20Multi-Domain%20Polymeric%20Microparticles%2C%20Materials%2012%20%282019%29%203921.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fma12233921%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fma12233921%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Preparation%20and%20Deep%20Characterization%20of%20Composite%5C%2FHybrid%20Multi-Scale%20and%20Multi-Domain%20Polymeric%20Microparticles%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Wei%22%2C%22lastName%22%3A%22Yu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nikunjkumar%22%2C%22lastName%22%3A%22Visaveliya%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Christophe%20A.%22%2C%22lastName%22%3A%22Serra%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22J.%20Michael%22%2C%22lastName%22%3A%22K%5Cu00f6hler%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Shukai%22%2C%22lastName%22%3A%22Ding%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Michel%22%2C%22lastName%22%3A%22Bouquey%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ren%5Cu00e9%22%2C%22lastName%22%3A%22Muller%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Marc%22%2C%22lastName%22%3A%22Schmutz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Isabelle%22%2C%22lastName%22%3A%22Kraus%22%7D%5D%2C%22abstractNote%22%3A%22Polymeric%20microparticles%20were%20produced%20following%20a%20three-step%20procedure%20involving%20%28i%29%20the%20production%20of%20an%20aqueous%20nanoemulsion%20of%20tri%20and%20monofunctional%20acrylate-based%20monomers%20droplets%20by%20an%20elongational-flow%20microemulsifier%2C%20%28ii%29%20the%20production%20of%20a%20nanosuspension%20upon%20the%20continuous-flow%20UV-initiated%20miniemulsion%20polymerization%20of%20the%20above%20nanoemulsion%20and%20%28iii%29%20the%20production%20of%20core-shell%20polymeric%20microparticles%20by%20means%20of%20a%20microfluidic%20capillaries-based%20double%20droplets%20generator%3B%20the%20core%20phase%20was%20composed%20of%20the%20above%20nanosuspension%20admixed%20with%20a%20water-soluble%20monomer%20and%20gold%20salt%2C%20the%20shell%20phase%20comprised%20a%20trifunctional%20monomer%2C%20diethylene%20glycol%20and%20a%20silver%20salt%3B%20both%20phases%20were%20photopolymerized%20on-the-fly%20upon%20droplet%20formation.%20Resulting%20microparticles%20were%20extensively%20analyzed%20by%20energy%20dispersive%20X-rays%20spectrometry%20and%20scanning%20electron%20microscopy%20to%20reveal%20the%20core-shell%20morphology%2C%20the%20presence%20of%20silver%20nanoparticles%20in%20the%20shell%2C%20organic%20nanoparticles%20in%20the%20core%20but%20failed%20to%20reveal%20the%20presence%20of%20the%20gold%20nanoparticles%20in%20the%20core%20presumably%20due%20to%20their%20too%20small%20size%20%28c.a.%202.5%20nm%29.%20Nevertheless%2C%20the%20reddish%20appearance%20of%20the%20as%20such%20prepared%20polymer%20microparticles%20emphasized%20that%20this%20three-step%20procedure%20allowed%20the%20easy%20elaboration%20of%20composite%5C%2Fhybrid%20multi-scale%20and%20multi-domain%20polymeric%20microparticles.%22%2C%22date%22%3A%222019%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.3390%5C%2Fma12233921%22%2C%22ISSN%22%3A%221996-1944%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.3390%5C%2Fma12233921%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22VRM2E3H6%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222021-04-30T13%3A45%3A25Z%22%7D%7D%2C%7B%22key%22%3A%227F3XASD3%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Shaya%20et%20al.%22%2C%22parsedDate%22%3A%222023%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EJ.%20Shaya%2C%20J.-C.%20Ribierre%2C%20G.%20Correia%2C%20Y.J.%20Dappe%2C%20F.%20Mathevet%2C%20L.%20Mager%2C%20B.%20Heinrich%2C%20S.%20M%26%23xE9%3Bry%2C%20Control%20of%20the%20Organization%20of%204%2C4%26%23x2019%3B-bis%28carbazole%29-1%2C1%26%23x2019%3B-biphenyl%20%28CBP%29%20Molecular%20Materials%20through%20Siloxane%20Functionalization.%2C%20Molecules%2028%20%282023%29%202038.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fmolecules28052038%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fmolecules28052038%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Control%20of%20the%20Organization%20of%204%2C4%27-bis%28carbazole%29-1%2C1%27-biphenyl%20%28CBP%29%20Molecular%20Materials%20through%20Siloxane%20Functionalization.%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Janah%22%2C%22lastName%22%3A%22Shaya%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jean-Charles%22%2C%22lastName%22%3A%22Ribierre%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Gabriel%22%2C%22lastName%22%3A%22Correia%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yannick%20J%22%2C%22lastName%22%3A%22Dappe%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Fabrice%22%2C%22lastName%22%3A%22Mathevet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Loic%22%2C%22lastName%22%3A%22Mager%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Benoit%22%2C%22lastName%22%3A%22Heinrich%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22St%5Cu00e9phane%22%2C%22lastName%22%3A%22M%5Cu00e9ry%22%7D%5D%2C%22abstractNote%22%3A%22We%20show%20that%20through%20the%20introduction%20of%20short%20dimethylsiloxane%20chains%2C%20it%20was%20possible%20to%20suppress%20the%20crystalline%20state%20of%20CBP%20in%20favor%20of%20various%20types%20of%20organization%2C%20transitioning%20from%20a%20soft%20crystal%20to%20a%20fluid%20liquid%20crystal%20mesophase%2C%20then%20to%20a%20liquid%20state.%20Characterized%20by%20X-ray%20scattering%2C%20all%20organizations%20reveal%20a%20similar%20layered%20configuration%20in%20which%20layers%20of%20edge-on%20lying%20CBP%20cores%20alternate%20with%20siloxane.%20The%20difference%20between%20all%20CBP%20organizations%20essentially%20lay%20on%20the%20regularity%20of%20the%20molecular%20packing%20that%20modulates%20the%20interactions%20of%20neighboring%20conjugated%20cores.%20As%20a%20result%2C%20the%20materials%20show%20quite%20different%20thin%20film%20absorption%20and%20emission%20properties%2C%20which%20could%20be%20correlated%20to%20the%20features%20of%20the%20chemical%20architectures%20and%20the%20molecular%20organizations.%22%2C%22date%22%3A%222023%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.3390%5C%2Fmolecules28052038%22%2C%22ISSN%22%3A%221420-3049%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.3390%5C%2Fmolecules28052038%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22CHW2VGSR%22%2C%22TK3HH32E%22%2C%22WWGPR7DV%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222023-05-31T13%3A25%3A16Z%22%7D%7D%2C%7B%22key%22%3A%22WRQBTKV2%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Shaya%20et%20al.%22%2C%22parsedDate%22%3A%222022%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EJ.%20Shaya%2C%20G.%20Correia%2C%20B.%20Heinrich%2C%20J.-C.%20Ribierre%2C%20K.%20Polychronopoulou%2C%20L.%20Mager%2C%20S.%20M%26%23xE9%3Bry%2C%20Functionalization%20of%20Biphenylcarbazole%20%28CBP%29%20with%20Siloxane-Hybrid%20Chains%20for%20Solvent-Free%20Liquid%20Materials%2C%20Molecules%2027%20%282022%29%2089.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fmolecules27010089%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fmolecules27010089%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Functionalization%20of%20Biphenylcarbazole%20%28CBP%29%20with%20Siloxane-Hybrid%20Chains%20for%20Solvent-Free%20Liquid%20Materials%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Janah%22%2C%22lastName%22%3A%22Shaya%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Gabriel%22%2C%22lastName%22%3A%22Correia%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Beno%5Cu00eet%22%2C%22lastName%22%3A%22Heinrich%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jean-Charles%22%2C%22lastName%22%3A%22Ribierre%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Kyriaki%22%2C%22lastName%22%3A%22Polychronopoulou%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Lo%5Cu00efc%22%2C%22lastName%22%3A%22Mager%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22St%5Cu00e9phane%22%2C%22lastName%22%3A%22M%5Cu00e9ry%22%7D%5D%2C%22abstractNote%22%3A%22We%20report%20herein%20the%20synthesis%20of%20siloxane-functionalized%20CBP%20molecules%20%284%2C4%26prime%3B-bis%28carbazole%29-1%2C1%26prime%3B-biphenyl%29%20for%20liquid%20optoelectronic%20applications.%20The%20room-temperature%20liquid%20state%20is%20obtained%20through%20a%20convenient%20functionalization%20of%20the%20molecules%20with%20heptamethyltrisiloxane%20chains%20via%20hydrosilylation%20of%20alkenyl%20spacers.%20The%20synthesis%20comprises%20screening%20of%20metal-catalyzed%20methodologies%20to%20introduce%20alkenyl%20linkers%20into%20carbazoles%20%28Stille%20and%20Suzuki%20Miyaura%20cross-couplings%29%2C%20incorporate%20the%20alkenylcarbazoles%20to%20dihalobiphenyls%20%28Ullmann%20coupling%29%2C%20and%20finally%20introduce%20the%20siloxane%20chains.%20The%20used%20conditions%20allowed%20the%20synthesis%20of%20the%20target%20compounds%2C%20despite%20the%20high%20reactivity%20of%20the%20alkenyl%20moieties%20bound%20to%20%26pi%3B-conjugated%20systems%20toward%20undesired%20side%20reactions%20such%20as%20polymerization%2C%20isomerization%2C%20and%20hydrogenation.%20The%20features%20of%20these%20solvent-free%20liquid%20CBP%20derivatives%20make%20them%20potentially%20interesting%20for%20fluidic%20optoelectronic%20applications.%22%2C%22date%22%3A%222022%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.3390%5C%2Fmolecules27010089%22%2C%22ISSN%22%3A%221420-3049%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.mdpi.com%5C%2F1420-3049%5C%2F27%5C%2F1%5C%2F89%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22CHW2VGSR%22%2C%22TK3HH32E%22%2C%22WWGPR7DV%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222022-03-22T16%3A09%3A18Z%22%7D%7D%2C%7B%22key%22%3A%22IG7VU4PD%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Serra%20et%20al.%22%2C%22parsedDate%22%3A%222013%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EC.A.%20Serra%2C%20I.U.%20Khan%2C%20Z.Q.%20Chang%2C%20M.%20Bouquey%2C%20R.%20Muller%2C%20I.%20Kraus%2C%20M.%20Schmutz%2C%20T.%20Vandamme%2C%20N.%20Anton%2C%20C.%20Ohm%2C%20R.%20Zentel%2C%20A.%20Knauer%2C%20M.%20Kohler%2C%20Engineering%20Polymer%20Microparticles%20by%20Droplet%20Microfluidics%2C%20Journal%20of%20Flow%20Chemistry%203%20%282013%29%2066%26%23x2013%3B75.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1556%5C%2Fjfc-d-13-00014%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1556%5C%2Fjfc-d-13-00014%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Engineering%20Polymer%20Microparticles%20by%20Droplet%20Microfluidics%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22C.%20A.%22%2C%22lastName%22%3A%22Serra%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22I.%20U.%22%2C%22lastName%22%3A%22Khan%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Z.%20Q.%22%2C%22lastName%22%3A%22Chang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22M.%22%2C%22lastName%22%3A%22Bouquey%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22R.%22%2C%22lastName%22%3A%22Muller%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22I.%22%2C%22lastName%22%3A%22Kraus%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22M.%22%2C%22lastName%22%3A%22Schmutz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22T.%22%2C%22lastName%22%3A%22Vandamme%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22N.%22%2C%22lastName%22%3A%22Anton%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22C.%22%2C%22lastName%22%3A%22Ohm%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22R.%22%2C%22lastName%22%3A%22Zentel%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22A.%22%2C%22lastName%22%3A%22Knauer%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22M.%22%2C%22lastName%22%3A%22Kohler%22%7D%5D%2C%22abstractNote%22%3A%22Capillary-based%20flow-focusing%20and%20co-flow%20microsystems%20were%20developed%20to%20produce%20sphere-like%20polymer%20microparticles%20of%20adjustable%20sizes%20in%20the%20range%20of%2050%20to%20600%20mu%20m%20with%20a%20narrow%20size%20distribution%20%28CV%20%3C%205%25%29%20and%20different%20morphologies%20%28core-shell%2C%20janus%2C%20and%20capsules%29.%20Rod-like%20particles%20whose%20length%20was%20conveniently%20adjusted%20between%20400%20mu%20m%20and%20few%20millimeters%20were%20also%20produced%20using%20the%20same%20microsystems.%20Influence%20of%20operating%20conditions%20%28flow%20rate%20of%20the%20different%20fluid%2C%20microsystem%20characteristic%20dimensions%2C%20and%20design%29%20as%20well%20as%20material%20parameters%20%28viscosity%20of%20the%20different%20fluids%20and%20surface%20tension%29%20was%20investigated.%20Empirical%20relationships%20were%20thus%20derived%20from%20experimental%20data%20to%20predict%20the%20microparticle%27s%20overall%20size%2C%20shell%20thickness%2C%20or%20rods%20length.%20Besides%20morphology%2C%20microparticles%20with%20various%20compositions%20were%20synthesized%20and%20their%20potential%20applications%20highlighted%3A%20drug-loaded%20microparticles%20for%20new%20drug%20delivery%20strategies%2C%20composed%20inorganic-organic%20multiscale%20microparticles%20for%20sensorics%2C%20and%20liquid%20crystalline%20elastomer%20microparticles%20showing%20an%20anisotropic%20reversible%20shape%20change%20upon%20temperature%20for%20thermal%20actuators%20or%20artificial%20muscles.%22%2C%22date%22%3A%222013%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1556%5C%2Fjfc-d-13-00014%22%2C%22ISSN%22%3A%222062-249X%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1556%5C%2Fjfc-d-13-00014%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22VRM2E3H6%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222021-04-30T13%3A44%3A45Z%22%7D%7D%2C%7B%22key%22%3A%223R4TMVGD%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Schwartz%20et%20al.%22%2C%22parsedDate%22%3A%222019%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EP.-O.%20Schwartz%2C%20F.%20Moingeon%2C%20J.%20Roeser%2C%20E.%20Couzigne%2C%20E.%20Voirin%2C%20P.%20Masson%2C%20S.%20M%26%23xE9%3Bry%2C%20Preparation%20of%20multi-allylic%20dendronized%20polymers%20via%20atom-transfer%20radical%20polymerization%2C%20European%20Polymer%20Journal%20118%20%282019%29%20358%26%23x2013%3B364.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.eurpolymj.2019.06.009%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.eurpolymj.2019.06.009%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Preparation%20of%20multi-allylic%20dendronized%20polymers%20via%20atom-transfer%20radical%20polymerization%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Pierre-Olivier%22%2C%22lastName%22%3A%22Schwartz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Firmin%22%2C%22lastName%22%3A%22Moingeon%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jerome%22%2C%22lastName%22%3A%22Roeser%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Emilie%22%2C%22lastName%22%3A%22Couzigne%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Emilie%22%2C%22lastName%22%3A%22Voirin%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Patrick%22%2C%22lastName%22%3A%22Masson%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22St%5Cu00e9phane%22%2C%22lastName%22%3A%22M%5Cu00e9ry%22%7D%5D%2C%22abstractNote%22%3A%22Atom-transfer%20radical%20polymerization%20%28ATRP%29%20was%20investigated%20to%20polymerize%20a%20styrene%20monomer%20carrying%20carbosilane%20dendrons%20with%206%20terminal%20allyl%20branches.%20Polymers%20with%20a%20monomodal%20molar%20mass%20distribution%20and%20low%20polydispersity%20have%20been%20produced%2C%20while%20by%20comparison%20the%20free-radical%20polymerization%20technique%20led%20to%20chain%20transfer%20early%20in%20the%20polymerization.%20Steric%20effect%20brought%20by%20the%20dendrons%20result%20in%20a%20slow%20polymerization%20rate%2C%20leading%20to%20an%20apparent%20saturation%20of%20the%20degree%20of%20polymerization.%20By%20pushing%20up%20the%20polymerization%20conditions%20%28eg.%20increase%20of%20temperature%20or%20concentration%29%2C%20interchain%20couplings%20started%20to%20take%20place%2C%20most%20likely%20from%20reactions%20at%20the%20allyl%20branches.%20These%20results%20are%20very%20similar%20to%20the%20ones%20previously%20reported%20for%20the%20anionic%20polymerization%20of%20this%20same%20multi-allylic%20dendronized%20monomer.%22%2C%22date%22%3A%222019%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.eurpolymj.2019.06.009%22%2C%22ISSN%22%3A%220014-3057%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1016%5C%2Fj.eurpolymj.2019.06.009%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22TK3HH32E%22%2C%22BMA9GKQT%22%2C%22VYTETDZF%22%2C%22IEGKATUQ%22%5D%2C%22dateModified%22%3A%222021-06-09T13%3A03%3A34Z%22%7D%7D%2C%7B%22key%22%3A%22IVM6Q2HB%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Schwartz%20et%20al.%22%2C%22parsedDate%22%3A%222013%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EP.O.%20Schwartz%2C%20E.%20Zaborova%2C%20R.%20Bechara%2C%20P.%20Leveque%2C%20T.%20Heiser%2C%20S.%20M%26%23xE9%3Bry%2C%20N.%20Leclerc%2C%20Impact%20of%20the%20arrangement%20of%20functional%20moieties%20within%20small%20molecular%20systems%20for%20solution%20processable%20bulk%20heterojunction%20solar%20cells%2C%20New%20Journal%20of%20Chemistry%2037%20%282013%29%202317%26%23x2013%3B2323.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fc3nj00218g%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fc3nj00218g%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Impact%20of%20the%20arrangement%20of%20functional%20moieties%20within%20small%20molecular%20systems%20for%20solution%20processable%20bulk%20heterojunction%20solar%20cells%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22P.%20O.%22%2C%22lastName%22%3A%22Schwartz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22E.%22%2C%22lastName%22%3A%22Zaborova%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22R.%22%2C%22lastName%22%3A%22Bechara%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22P.%22%2C%22lastName%22%3A%22Leveque%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22T.%22%2C%22lastName%22%3A%22Heiser%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22St%5Cu00e9phane%22%2C%22lastName%22%3A%22M%5Cu00e9ry%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22N.%22%2C%22lastName%22%3A%22Leclerc%22%7D%5D%2C%22abstractNote%22%3A%22A%20series%20of%20molecules%20based%20on%20thienofluorene%20derivatives%20as%20electron%20donating%20%28D%29%20and%20benzothiadiazole%20as%20electron%20accepting%20%28A%29%20moieties%20have%20been%20assembled%20into%20DAD%20and%20ADA%20architectures%20in%20order%20to%20investigate%20the%20influence%20of%20the%20way%20of%20assembling%20the%20D%20and%20A%20units%20along%20the%20conjugated%20backbone%2C%20on%20the%20photovoltaic%20performances%20of%20bulk%20heterojunction%20solar%20cells.%20It%20was%20found%20that%20the%20major%20difference%20in%20going%20from%20ADA%20to%20DAD%20architecture%20is%20the%20change%20in%20molecular%20organization%20%28nematic%20to%20crystalline%29%2C%20which%20increases%20the%20charge%20transport%20mobility%20and%20probably%20also%20affects%20the%20structural%20organization%20in%20blends%20with%20PCBM%20that%20ultimately%20leads%20to%20higher%20photovoltaic%20performances.%22%2C%22date%22%3A%222013%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1039%5C%2Fc3nj00218g%22%2C%22ISSN%22%3A%221144-0546%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1039%5C%2Fc3nj00218g%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22BMA9GKQT%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222021-06-09T13%3A00%3A46Z%22%7D%7D%2C%7B%22key%22%3A%22EGJTZBDM%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Schwartz%20et%20al.%22%2C%22parsedDate%22%3A%222014%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EP.-O.%20Schwartz%2C%20L.%20Biniek%2C%20E.%20Zaborova%2C%20B.%20Heinrich%2C%20M.%20Brinkrnann%2C%20N.%20Leclerc%2C%20S.%20M%26%23xE9%3Bry%2C%20Perylenediimide-Based%20Donor-Acceptor%20Dyads%20and%20Triads%3A%20Impact%20of%20Molecular%20Architecture%20on%20Self-Assembling%20Properties%2C%20Journal%20of%20the%20American%20Chemical%20Society%20136%20%282014%29%205981%26%23x2013%3B5992.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1021%5C%2Fja4129108%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1021%5C%2Fja4129108%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Perylenediimide-Based%20Donor-Acceptor%20Dyads%20and%20Triads%3A%20Impact%20of%20Molecular%20Architecture%20on%20Self-Assembling%20Properties%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Pierre-Olivier%22%2C%22lastName%22%3A%22Schwartz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Laure%22%2C%22lastName%22%3A%22Biniek%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Elena%22%2C%22lastName%22%3A%22Zaborova%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Benoit%22%2C%22lastName%22%3A%22Heinrich%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Martin%22%2C%22lastName%22%3A%22Brinkrnann%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nicolas%22%2C%22lastName%22%3A%22Leclerc%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22St%5Cu00e9phane%22%2C%22lastName%22%3A%22M%5Cu00e9ry%22%7D%5D%2C%22abstractNote%22%3A%22Perylenediimide-based%20donor%20acceptor%20cooligomers%20are%20particularly%20attractive%20in%20plastic%20electronics%20because%20of%20their%20unique%20electro-active%20properties%20that%20can%20be%20tuned%20by%20proper%20chemical%20engineering.%20Herein%2C%20a%20new%20class%20of%20co-oligomers%20has%20been%20synthesized%20with%20a%20dyad%20structure%20%28AD%29%20or%20a%20triad%20structure%20%28DAD%20and%20ADA%29%20in%20order%20to%20understand%20the%20correlations%20between%20the%20co-oligomer%20molecular%20architecture%20and%20the%20structures%20formed%20by%20self-assembly%20in%20thin%20films.%20The%20acceptor%20block%20A%20is%20a%20perylene%20tetracarboxyl%20diimide%20%28PDI%29%2C%20whereas%20the%20donor%20block%20D%20is%20made%20of%20a%20combination%20of%20thiophene%2C%20fluorene%2C%20and%202%2C1%2C3-benzothiadiazole%20derivatives.%20D%20and%20A%20blocks%20are%20linked%20by%20a%20short%20and%20flexible%20ethylene%20spacer%20to%20ease%20self-assembling%20in%20thin%20films.%20Structural%20studies%20using%20small%20and%20wide%20X-ray%20diffraction%20and%20transmission%20electron%20microscopy%20demonstrate%20that%20AD%20and%20ADA%20lamellae%20are%20made%20of%20a%20double%20layer%20of%20co-oligomers%20with%20overlapping%20and%20strongly%20pi-stacked%20PDI%20units%20because%20the%20sectional%20area%20of%20the%20PDI%20is%20about%20half%20that%20of%20the%20donor%20block.%20These%20structural%20models%20allow%20rationalizing%20the%20absence%20of%20organization%20for%20the%20DAD%20co-oligomer%20and%20therefore%20to%20draw%20general%20rules%20for%20the%20design%20of%20PDI-based%20dyads%20and%20triads%20with%20proper%20self-assembling%20properties%20of%20use%20in%20organic%20electronics.%22%2C%22date%22%3A%222014%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1021%5C%2Fja4129108%22%2C%22ISSN%22%3A%220002-7863%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1021%5C%2Fja4129108%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22TK3HH32E%22%2C%22BMA9GKQT%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222021-06-09T13%3A00%3A54Z%22%7D%7D%2C%7B%22key%22%3A%22P34SP8SW%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Roland%20et%20al.%22%2C%22parsedDate%22%3A%222011%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ET.%20Roland%2C%20G.H.%20Ramirez%2C%20J.%20L%26%23xE9%3Bonard%2C%20S.%20M%26%23xE9%3Bry%2C%20S.%20Haacke%2C%20Ultrafast%20broadband%20laser%20spectroscopy%20reveals%20energy%20and%20charge%20transfer%20in%20novel%20donor-acceptor%20triads%20for%20photovoltaic%20applications%2C%20in%3A%20Ye%2C%20C%20and%20Wang%2C%20ZL%20and%20Zhou%2C%20B%20%28Ed.%29%2C%20Journal%20of%20Physics%3A%20Conference%20Series%2C%202011%3A%20p.%20012006%20%5C%2Fp.%201%26%23x2013%3B6.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1088%5C%2F1742-6596%5C%2F276%5C%2F1%5C%2F012006%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1088%5C%2F1742-6596%5C%2F276%5C%2F1%5C%2F012006%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22conferencePaper%22%2C%22title%22%3A%22Ultrafast%20broadband%20laser%20spectroscopy%20reveals%20energy%20and%20charge%20transfer%20in%20novel%20donor-acceptor%20triads%20for%20photovoltaic%20applications%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thomas%22%2C%22lastName%22%3A%22Roland%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22G.%20Hernandez%22%2C%22lastName%22%3A%22Ramirez%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22J%5Cu00e9r%5Cu00e9mie%22%2C%22lastName%22%3A%22L%5Cu00e9onard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22St%5Cu00e9phane%22%2C%22lastName%22%3A%22M%5Cu00e9ry%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stefan%22%2C%22lastName%22%3A%22Haacke%22%7D%2C%7B%22creatorType%22%3A%22editor%22%2C%22name%22%3A%22Ye%2C%20C%20and%20Wang%2C%20ZL%20and%20Zhou%2C%20B%22%7D%5D%2C%22abstractNote%22%3A%22Triggered%20by%20the%20quest%20for%20new%20organic%20materials%20and%20micro-structures%20for%20photovoltaic%20applications%2C%20a%20novel%20class%20of%20donor-acceptor-donor%20%28DAD%29%20triads%20extended%20with%20siloxane%20chains%20has%20been%20synthesized%20in%20our%20labs.%20Because%20of%20the%20siloxane%20chains%2C%20the%20molecules%20self-organize%20into%20a%20smectic%20liquid%20crystal%20phase%2C%20resulting%20in%20a%20stacking%20of%20the%20DAD%20cores.%20We%20report%20here%20a%20preliminary%20study%20of%20the%20ultrafast%20dynamics%20of%20energy%20and%20charge%20transfer%20studied%20by%20femtosecond%20broadband%20transient%20absorption%20experiments%20on%20isolated%20triads%20in%20chloroform.%22%2C%22date%22%3A%222011%22%2C%22proceedingsTitle%22%3A%22Journal%20of%20Physics%3A%20Conference%20Series%22%2C%22conferenceName%22%3A%223rd%20International%20Photonics%20and%20Optoelectronics%20Meeting%20%28POEM%202010%29%3B%20Wuhan%2C%20Chine%2C%203-5%20Novembre%202010%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1088%5C%2F1742-6596%5C%2F276%5C%2F1%5C%2F012006%22%2C%22ISBN%22%3A%22%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1088%5C%2F1742-6596%5C%2F276%5C%2F1%5C%2F012006%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22CHW2VGSR%22%2C%2295EJ8IDX%22%2C%22BMA9GKQT%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222021-06-09T13%3A00%3A22Z%22%7D%7D%2C%7B%22key%22%3A%22Q92T7N7F%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Roland%20et%20al.%22%2C%22parsedDate%22%3A%222012%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ET.%20Roland%2C%20J.%20L%26%23xE9%3Bonard%2C%20G.H.%20Ramirez%2C%20S.%20M%26%23xE9%3Bry%2C%20O.%20Yurchenko%2C%20S.%20Ludwigs%2C%20S.%20Haacke%2C%20Sub-100%20fs%20charge%20transfer%20in%20a%20novel%20donor-acceptor-donor%20triad%20organized%20in%20a%20smectic%20film%2C%20Physical%20Chemistry%20Chemical%20Physics%2014%20%282012%29%20273%26%23x2013%3B279.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fc1cp22122a%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fc1cp22122a%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Sub-100%20fs%20charge%20transfer%20in%20a%20novel%20donor-acceptor-donor%20triad%20organized%20in%20a%20smectic%20film%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thomas%22%2C%22lastName%22%3A%22Roland%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22J%5Cu00e9r%5Cu00e9mie%22%2C%22lastName%22%3A%22L%5Cu00e9onard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22G.%20Hernandez%22%2C%22lastName%22%3A%22Ramirez%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22St%5Cu00e9phane%22%2C%22lastName%22%3A%22M%5Cu00e9ry%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22O.%22%2C%22lastName%22%3A%22Yurchenko%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22S.%22%2C%22lastName%22%3A%22Ludwigs%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stefan%22%2C%22lastName%22%3A%22Haacke%22%7D%5D%2C%22abstractNote%22%3A%22Ultrafast%20transient%20absorption%20spectroscopy%20is%20performed%20on%20a%20novel%20donor-acceptor-donor%20triad%20made%20of%20two%20identical%20bisthiophene%20derivatives%20as%20electron%20donors%20and%20a%20central%20perylenediimide%20moiety%20as%20electron%20acceptor.%20The%20triad%20is%20extended%20at%20both%20ends%20by%20covalently%20bound%20siloxane%20chains%20that%20confer%20self-organisation%20into%20thin%20smectic%20films%20at%20ambient%20temperature.%20When%20diluted%20in%20chloroform%2C%20selective%20excitation%20of%20the%20donor%20moiety%20leads%20to%20resonance%20energy%20transfer%20within%20130%20fs%20to%20the%20acceptor%20moiety%2C%20followed%20by%20the%20formation%20of%20a%20charge%20transfer%20%28CT%29%20state%20in%20similar%20to%203%20ps.%20The%20CT%20state%20recombines%20entirely%20on%20a%2055%20ps%20time%20scale.%20In%20the%20liquid%20crystal%20films%2C%20excitonic%20intermolecular%20coupling%20leads%20to%20significant%20changes%20in%20the%20dynamics.%20Most%20remarkably%2C%20ultrafast%20intra-and%20intermolecular%20CT%20state%20formation%20occurs%20in%20about%2060%20fs%2C%20i.e.%20on%20a%20time%20scale%20comparable%20to%20electronic%20coherence%20times.%20While%20the%20intra-molecular%20CT%20states%20recombine%20on%20the%20same%20time%20scale%20as%20in%20solution%20or%20even%20faster%2C%20inter-molecular%20CT%20states%20live%20for%20about%201%20ns.%20Last%2C%20triplet%20states%20of%20the%20perylenediimide%20moiety%20dominate%20the%20differential%20absorption%20after%20similar%20to%201%20ns.%20We%20anticipate%20that%20the%20fast%20recombination%20of%20intra-molecular%20CT%20states%20and%20the%20triplet%20state%20formation%20may%20severely%20limit%20the%20photo-current%20in%20these%20materials.%22%2C%22date%22%3A%222012%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1039%5C%2Fc1cp22122a%22%2C%22ISSN%22%3A%221463-9076%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1039%5C%2Fc1cp22122a%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22CHW2VGSR%22%2C%2295EJ8IDX%22%2C%22BMA9GKQT%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222021-06-09T13%3A00%3A30Z%22%7D%7D%2C%7B%22key%22%3A%22FJ3TJJVW%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Roeser%20et%20al.%22%2C%22parsedDate%22%3A%222011%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EJ.%20Roeser%2C%20F.%20Moingeon%2C%20B.%20Heinrich%2C%20P.%20Masson%2C%20F.%20Arnaud-Neu%2C%20M.%20Rawiso%2C%20S.%20M%26%23xE9%3Bry%2C%20Dendronized%20Polymers%20with%20Peripheral%20Oligo%28ethylene%20oxide%29%20Chains%3A%20Thermoresponsive%20Behavior%20and%20Shape%20Anisotropy%20in%20Solution%2C%20Macromolecules%2044%20%282011%29%208925%26%23x2013%3B8935.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1021%5C%2Fma2016776%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1021%5C%2Fma2016776%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Dendronized%20Polymers%20with%20Peripheral%20Oligo%28ethylene%20oxide%29%20Chains%3A%20Thermoresponsive%20Behavior%20and%20Shape%20Anisotropy%20in%20Solution%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jerome%22%2C%22lastName%22%3A%22Roeser%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Firmin%22%2C%22lastName%22%3A%22Moingeon%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Benoit%22%2C%22lastName%22%3A%22Heinrich%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Patrick%22%2C%22lastName%22%3A%22Masson%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Francoise%22%2C%22lastName%22%3A%22Arnaud-Neu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Michel%22%2C%22lastName%22%3A%22Rawiso%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22M%5Cu00e9ry%22%7D%5D%2C%22abstractNote%22%3A%22A%20series%20of%20dendronized%20polymers%20carrying%20oligo%28ethyleneoxy%29%20peripheral%20branches%20have%20been%20prepared%20by%20postpolymerization%20functionalization%20of%20multiallylic%20dendronized%20polymers%20using%20a%20radical%20addition%20of%20mercaptans%2C%20namely%202-methoxy%28ethoxy%29ethanethiol%20%28EO2%29%20and%20%7B2-%282-methoxyethoxy%29ethoxy%29%7D%20ethanethiol%20%28EO3%29.%20The%20functionalization%20proved%20to%20be%20quite%20efficient%2C%20leading%20to%20up%20to%206%20or%209%20EO%20chains%20per%20monomer%20repeating%20unit.%20A%20constant%20T%28g%29%20value%20was%20observed%20independently%20of%20the%20material%20characteristics%2C%20indicating%20that%20T%28g%29%20is%20ruled%20by%20the%20sole%20presence%20of%20EO%20chains.%20According%20to%20the%20hydrophilic%20and%20hydrophobic%20balance%2C%20some%20polymers%20exhibited%20a%20thermoresponsive%20behavior%20in%20water%20solution%2C%20characterized%20by%20a%20sharp%20lower%20critical%20solution%20temperature%20%28LCST%29%20transition%20and%20a%20small%20hysteresis.%20These%20LCST%20showed%20an%20unusual%20increase%20with%20DP%2C%20which%20might%20be%20correlated%20to%20a%20dilution%20effect%20and%20an%20increase%20of%20polymer%20hydrophilicity%20by%20densification%20of%20the%20dendritic%20coverage.%20By%20SAXS%20investigations%20and%20using%20a%20spherocylinder%20shape%20model%2C%20the%20polymers%20in%20solution%20%28below%20the%20LCST%29%20could%20be%20satisfactorily%20described.%20By%20increasing%20the%20DP%2C%20the%20shape%20of%20the%20macromolecule%20was%20found%20to%20evolve%20from%20a%20spherical%20to%20a%20spherocylinder%20shape%20with%20a%20constant%20cross%20section%20of%20ca.%2040%20angstrom.%22%2C%22date%22%3A%222011%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1021%5C%2Fma2016776%22%2C%22ISSN%22%3A%220024-9297%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1021%5C%2Fma2016776%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22TK3HH32E%22%2C%22BMA9GKQT%22%2C%22VYTETDZF%22%2C%22IEGKATUQ%22%5D%2C%22dateModified%22%3A%222021-06-09T13%3A00%3A15Z%22%7D%7D%2C%7B%22key%22%3A%22THEDKRVE%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Roeser%20et%20al.%22%2C%22parsedDate%22%3A%222013%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EJ.%20Roeser%2C%20B.%20Heinrich%2C%20C.%20Bourgogne%2C%20M.%20Rawiso%2C%20S.%20Michel%2C%20V.%20Hubscher-Bruder%2C%20F.%20Arnaud-Neu%2C%20S.%20M%26%23xE9%3Bry%2C%20Dendronized%20Polymers%20with%20Silver%20and%20Mercury%20Cations%20Recognition%3A%20Complexation%20Studies%20and%20Polyelectrolyte%20Behavior%2C%20Macromolecules%20%28Washington%2C%20DC%2C%20U.%20S.%29%2046%20%282013%29%207075%26%23x2013%3B7085.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1021%5C%2Fma400348v%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1021%5C%2Fma400348v%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Dendronized%20Polymers%20with%20Silver%20and%20Mercury%20Cations%20Recognition%3A%20Complexation%20Studies%20and%20Polyelectrolyte%20Behavior%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jerome%22%2C%22lastName%22%3A%22Roeser%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Benoit%22%2C%22lastName%22%3A%22Heinrich%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Cyril%22%2C%22lastName%22%3A%22Bourgogne%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Michel%22%2C%22lastName%22%3A%22Rawiso%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sylvia%22%2C%22lastName%22%3A%22Michel%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Veronique%22%2C%22lastName%22%3A%22Hubscher-Bruder%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Francoise%22%2C%22lastName%22%3A%22Arnaud-Neu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22St%5Cu00e9phane%22%2C%22lastName%22%3A%22M%5Cu00e9ry%22%7D%5D%2C%22abstractNote%22%3A%22Metal%20binding%20properties%20of%20a%20series%20of%20worm-like%20dendronized%20polymers%20bearing%20oxathiaether-based%20dendrons%20are%20reported.%20Extensive%20characterization%20of%20the%20complexation%20properties%20toward%20a%20large%20range%20of%20metal%20cations%20showed%20a%20high%20and%20selective%20affinity%20of%20the%20polymers%20for%20Ag%2B%20and%20Hg2%2B%20cations.%20Its%20origin%20is%20explained%20by%20the%20presence%20of%20specific%20M%5Cu00b7%5Cu00b7%5Cu00b7S%20and%20M%5Cu00b7%5Cu00b7%5Cu00b7O%20interactions%20%28M%20%3D%20Ag%2B%20or%20Hg2%2B%29%20within%20a%20cage%20structure%20formed%20by%20the%20dendritic%20moiety.%20The%20stoichiometry%20of%20the%20complexation%20is%20found%20to%20be%20affected%20by%20the%20degree%20of%20steric%20constraints%20in%20the%20dendronized%20materials.%20The%20effect%20of%20Ag%2B%20complexation%20leads%20to%20the%20appearance%20of%20polyelectrolyte%5C%2Fcharged%20colloid%20properties%20which%20were%20intensively%20studied%20by%20SANS.%20A%20significant%20result%20is%20the%20absence%20of%20major%20modification%20of%20the%20%28spherocylinder%29%20shape%20of%20the%20polymers%20upon%20Ag%2B%20sequestration%20which%20confirms%20the%20above%20mentioned%20complexation%20scenario.%20Another%20outstanding%20result%20of%20Ag%2B%20complexation%20is%20the%20Coulombic%20stabilization%20of%20the%20charged%20denpols%20that%20drastically%20affects%20their%20thermoresponsive%20properties%20%28sharp%20elevation%20of%20LCST%29%2C%20indicating%20possible%20chemosensing%20applications.%20%5Bon%20SciFinder%28R%29%5D%22%2C%22date%22%3A%222013%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1021%5C%2Fma400348v%22%2C%22ISSN%22%3A%220024-9297%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1021%5C%2Fma400348v%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22TK3HH32E%22%2C%22BMA9GKQT%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222021-06-09T13%3A00%3A39Z%22%7D%7D%2C%7B%22key%22%3A%22IQFR9XRT%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Ribierre%20et%20al.%22%2C%22parsedDate%22%3A%222019%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EJ.-C.%20Ribierre%2C%20Z.%20Li%2C%20X.%20Liu%2C%20E.%20Lacaze%2C%20B.%20Heinrich%2C%20S.%20M%26%23xE9%3Bry%2C%20P.%20Sleczkowski%2C%20Y.%20Xiao%2C%20F.%20Lafolet%2C%20D.%20Hashizume%2C%20T.%20Aoyama%2C%20M.%20Uchiyama%2C%20J.W.%20Wu%2C%20E.%20Zaborova%2C%20F.%20Fages%2C%20A.%20D%26%23x2019%3BAleo%2C%20F.%20Mathevet%2C%20C.%20Adachi%2C%20A%20solvent-free%20and%20vacuum-free%20melt-processing%20method%20to%20fabricate%20organic%20semiconducting%20layers%20with%20large%20crystal%20size%20for%20organic%20electronic%20applications%2C%20J.%20Mater.%20Chem.%20C%20%282019%29.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2FC8TC04834G%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2FC8TC04834G%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22A%20solvent-free%20and%20vacuum-free%20melt-processing%20method%20to%20fabricate%20organic%20semiconducting%20layers%20with%20large%20crystal%20size%20for%20organic%20electronic%20applications%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jean-Charles%22%2C%22lastName%22%3A%22Ribierre%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Zhao%22%2C%22lastName%22%3A%22Li%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Xiao%22%2C%22lastName%22%3A%22Liu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Emmanuelle%22%2C%22lastName%22%3A%22Lacaze%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Beno%5Cu00eet%22%2C%22lastName%22%3A%22Heinrich%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22St%5Cu00e9phane%22%2C%22lastName%22%3A%22M%5Cu00e9ry%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Piotr%22%2C%22lastName%22%3A%22Sleczkowski%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yiming%22%2C%22lastName%22%3A%22Xiao%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Fr%5Cu00e9d%5Cu00e9ric%22%2C%22lastName%22%3A%22Lafolet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Daisuke%22%2C%22lastName%22%3A%22Hashizume%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Tetsuya%22%2C%22lastName%22%3A%22Aoyama%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Masanobu%22%2C%22lastName%22%3A%22Uchiyama%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jeong%20Weon%22%2C%22lastName%22%3A%22Wu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Elena%22%2C%22lastName%22%3A%22Zaborova%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Fr%5Cu00e9d%5Cu00e9ric%22%2C%22lastName%22%3A%22Fages%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Anthony%22%2C%22lastName%22%3A%22D%27Aleo%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Fabrice%22%2C%22lastName%22%3A%22Mathevet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Chihaya%22%2C%22lastName%22%3A%22Adachi%22%7D%5D%2C%22abstractNote%22%3A%22We%20report%20on%20an%20improved%20melt-processing%20method%20to%20prepare%20organic%20semiconducting%20layers%20with%20large%20crystal%20size%20and%20enhanced%20charge%20carrier%20mobilities.%20The%20organic%20compound%20used%20in%20this%20work%20is%20a%20solution-processable%20oligo%28p-phenylene%20vinylene%29%20derivative%20substituted%20at%20both%20ends%20with%20pyrene%20moieties.%20Accurate%20control%20of%20the%20temperature%20during%20the%20recrystallization%20of%20this%20compound%20from%20the%20melt%20enables%20the%20formation%20of%20large%20single%20crystal%20monodomains%20in%20thin%20films.%20The%20melt-processed%20organic%20layer%20shows%20higher%20mobilities%20in%20transistor%20configuration%20than%20in%20spin-coated%20films%2C%20which%20can%20be%20attributed%20to%20the%20presence%20of%20large-size%20crystalline%20monodomains%20as%20evidenced%20by%20X-ray%20diffraction%20measurements.%20We%20also%20investigated%20the%20photophysical%20properties%20of%20this%20material%20in%20spin-coated%20and%20melted%20films%20and%20found%20an%20increase%20of%20the%20photoluminescence%20quantum%20yield%20with%20the%20size%20of%20the%20crystals%20in%20the%20organic%20layer.%20The%20advantage%20of%20this%20method%20over%20the%20spin%20coating%20also%20allowed%20observation%20of%20amplified%20spontaneous%20emission%20that%20was%20only%20achieved%20in%20the%20melted%20film%20due%20to%20its%20improved%20luminescence%20efficiency.%20Overall%2C%20this%20study%20demonstrates%20a%20simple%20and%20versatile%20method%2C%20which%20does%20not%20require%20the%20use%20of%20any%20solvent%20and%20vacuum%2C%20to%20fabricate%20organic%20layers%20with%20large%20crystal%20size%2C%20suitable%20for%20the%20realization%20of%20organic%20electronic%20and%20light-emitting%20devices.%22%2C%22date%22%3A%222019%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1039%5C%2FC8TC04834G%22%2C%22ISSN%22%3A%222050-7526%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1039%5C%2FC8TC04834G%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22TK3HH32E%22%2C%22BMA9GKQT%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222021-11-17T14%3A38%3A56Z%22%7D%7D%2C%7B%22key%22%3A%22ENDUXX4Q%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Ribierre%20et%20al.%22%2C%22parsedDate%22%3A%222018%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EJ.-C.%20Ribierre%2C%20T.%20Tanaka%2C%20L.%20Zhao%2C%20Y.%20Yokota%2C%20S.%20Matsumoto%2C%20D.%20Hashizume%2C%20K.%20Takaishi%2C%20T.%20Muto%2C%20B.%20Heinrich%2C%20S.%20M%26%23xE9%3Bry%2C%20F.%20Mathevet%2C%20T.%20Matsushima%2C%20M.%20Uchiyama%2C%20C.%20Adachi%2C%20T.%20Aoyama%2C%20Simultaneous%20Edge-on%20to%20Face-on%20Reorientation%20and%201D%20Alignment%20of%20Small%20pi-Conjugated%20Molecules%20Using%20Room-Temperature%20Mechanical%20Rubbing%2C%20Advanced%20Functional%20Materials%2028%20%282018%29.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fadfm.201707038%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fadfm.201707038%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Simultaneous%20Edge-on%20to%20Face-on%20Reorientation%20and%201D%20Alignment%20of%20Small%20pi-Conjugated%20Molecules%20Using%20Room-Temperature%20Mechanical%20Rubbing%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jean-Charles%22%2C%22lastName%22%3A%22Ribierre%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Toshihiko%22%2C%22lastName%22%3A%22Tanaka%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Li%22%2C%22lastName%22%3A%22Zhao%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yuki%22%2C%22lastName%22%3A%22Yokota%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Shinya%22%2C%22lastName%22%3A%22Matsumoto%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Daisuke%22%2C%22lastName%22%3A%22Hashizume%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Kazuto%22%2C%22lastName%22%3A%22Takaishi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Tsuyoshi%22%2C%22lastName%22%3A%22Muto%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Benoit%22%2C%22lastName%22%3A%22Heinrich%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22St%5Cu00e9phane%22%2C%22lastName%22%3A%22M%5Cu00e9ry%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Fabrice%22%2C%22lastName%22%3A%22Mathevet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Toshinori%22%2C%22lastName%22%3A%22Matsushima%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Masanobu%22%2C%22lastName%22%3A%22Uchiyama%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Chihaya%22%2C%22lastName%22%3A%22Adachi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Tetsuya%22%2C%22lastName%22%3A%22Aoyama%22%7D%5D%2C%22abstractNote%22%3A%22In%20this%20study%2C%20room-temperature%20mechanical%20rubbing%20is%20used%20to%20control%20the%203D%20orientation%20of%20small%20-conjugated%20molecular%20systems%20in%20solution-processed%20polycrystalline%20thin%20films%20without%20using%20any%20alignment%20substrate.%20High%20absorption%20dichroic%20ratio%20and%20significant%20anisotropy%20in%20charge%20carrier%20mobilities%20%28up%20to%20130%29%20measured%20in%20transistor%20configuration%20are%20obtained%20in%20rubbed%20organic%20films%20based%20on%20the%20ambipolar%20quinoidal%20quaterthiophene%20%28QQT%28CN%294%29.%20Moreover%2C%20a%20solvent%20vapor%20annealing%20treatment%20of%20the%20rubbed%20film%20is%20found%20to%20improve%20the%20optical%20and%20charge%20transport%20anisotropy%20due%20to%20an%20increased%20crystallinity.%20X-ray%20diffraction%20and%20atomic%20force%20microscopy%20measurements%20demonstrate%20that%20rubbing%20does%20not%20only%20lead%20to%20an%20excellent%201D%20orientation%20of%20the%20QQT%28CN%294%20molecules%20over%20large%20areas%20but%20also%20modifies%20the%20orientation%20of%20the%20crystals%2C%20moving%20molecules%20from%20an%20edge-on%20to%20a%20face-on%20configuration.%20The%20reasons%20why%20a%20mechanical%20alignment%20technique%20can%20be%20used%20at%20room%20temperature%20for%20such%20a%20polycrystalline%20film%20are%20rationalized%2C%20by%20the%20plastic%20characteristics%20of%20the%20QQT%28CN%294%20layer%20and%20the%20role%20of%20the%20flexible%20alkyl%20side%20chains%20in%20the%20molecular%20packing.%20This%20nearly%20complete%20conversion%20from%20edge-on%20to%20face-on%20orientation%20by%20mechanical%20treatment%20in%20polycrystalline%20small-molecule-based%20thin%20films%20opens%20perspectives%20in%20terms%20of%20fundamental%20research%20and%20practical%20applications%20in%20organic%20optoelectronics.%22%2C%22date%22%3A%222018%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1002%5C%2Fadfm.201707038%22%2C%22ISSN%22%3A%221616-301X%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1002%5C%2Fadfm.201707038%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22BMA9GKQT%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222021-06-09T13%3A03%3A10Z%22%7D%7D%2C%7B%22key%22%3A%2245UXJ28Q%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Ribierre%20et%20al.%22%2C%22parsedDate%22%3A%222016%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EJ.-C.%20Ribierre%2C%20L.%20Zhao%2C%20M.%20Inoue%2C%20P.-O.%20Schwartz%2C%20J.-H.%20Kim%2C%20K.%20Yoshida%2C%20A.S.D.%20Sandanayaka%2C%20H.%20Nakanotani%2C%20L.%20Mager%2C%20S.%20M%26%23xE9%3Bry%2C%20C.%20Adachi%2C%20Low%20threshold%20amplified%20spontaneous%20emission%20and%20ambipolar%20charge%20transport%20in%20non-volatile%20liquid%20fluorene%20derivatives%2C%20Chemical%20Communications%2052%20%282016%29%203103%26%23x2013%3B3106.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fc5cc08331a%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fc5cc08331a%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Low%20threshold%20amplified%20spontaneous%20emission%20and%20ambipolar%20charge%20transport%20in%20non-volatile%20liquid%20fluorene%20derivatives%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jean-Charles%22%2C%22lastName%22%3A%22Ribierre%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Li%22%2C%22lastName%22%3A%22Zhao%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Munetomo%22%2C%22lastName%22%3A%22Inoue%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Pierre-Olivier%22%2C%22lastName%22%3A%22Schwartz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ju-Hyung%22%2C%22lastName%22%3A%22Kim%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Kou%22%2C%22lastName%22%3A%22Yoshida%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Atula%20S.%20D.%22%2C%22lastName%22%3A%22Sandanayaka%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Hajime%22%2C%22lastName%22%3A%22Nakanotani%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Loic%22%2C%22lastName%22%3A%22Mager%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22St%5Cu00e9phane%22%2C%22lastName%22%3A%22M%5Cu00e9ry%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Chihaya%22%2C%22lastName%22%3A%22Adachi%22%7D%5D%2C%22abstractNote%22%3A%22Highly%20fluorescent%20non-volatile%20fluidic%20fluorene%20derivatives%20functionalized%20with%20siloxane%20chains%20were%20synthesized%20and%20used%20in%20monolithic%20solvent-free%20liquid%20organic%20semiconductor%20distributed%20feedback%20lasers.%20The%20photoluminescence%20quantum%20yield%20values%2C%20the%20amplified%20spontaneous%20emission%20thresholds%20and%20the%20ambipolar%20charge%20carrier%20mobilities%20demonstrate%20that%20this%20class%20of%20materials%20is%20extremely%20promising%20for%20organic%20fluidic%20light-emitting%20and%20lasing%20devices.%22%2C%22date%22%3A%222016%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1039%5C%2Fc5cc08331a%22%2C%22ISSN%22%3A%221359-7345%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1039%5C%2Fc5cc08331a%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22CHW2VGSR%22%2C%22WWGPR7DV%22%2C%22BMA9GKQT%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222021-06-09T13%3A02%3A27Z%22%7D%7D%2C%7B%22key%22%3A%224SERCAWQ%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Polkehn%20et%20al.%22%2C%22parsedDate%22%3A%222016%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EM.%20Polkehn%2C%20P.%20Eisenbrandt%2C%20H.%20Tamura%2C%20S.%20Haacke%2C%20S.%20M%26%23xE9%3Bry%2C%20I.%20Burghardt%2C%20Ultrafast%20excitonic%20and%20charge%20transfer%20dynamics%20in%20nanostructured%20organic%20polymer%20materials%2C%20in%3A%20Andrews%2C%20DL%20and%20Nunzi%2C%20JM%20and%20Ostendorf%2C%20A%20%28Ed.%29%2C%20NANOPHOTONICS%20VI%2C%20SPIE-INT%20SOC%20OPTICAL%20ENGINEERING%2C%201000%2020TH%20ST%2C%20PO%20BOX%2010%2C%20BELLINGHAM%2C%20WA%2098227-0010%20USA%2C%202016.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1117%5C%2F12.2230314%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1117%5C%2F12.2230314%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22conferencePaper%22%2C%22title%22%3A%22Ultrafast%20excitonic%20and%20charge%20transfer%20dynamics%20in%20nanostructured%20organic%20polymer%20materials%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matthias%22%2C%22lastName%22%3A%22Polkehn%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Pierre%22%2C%22lastName%22%3A%22Eisenbrandt%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Hiroyuki%22%2C%22lastName%22%3A%22Tamura%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stefan%22%2C%22lastName%22%3A%22Haacke%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22St%5Cu00e9phane%22%2C%22lastName%22%3A%22M%5Cu00e9ry%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Irene%22%2C%22lastName%22%3A%22Burghardt%22%7D%2C%7B%22creatorType%22%3A%22editor%22%2C%22name%22%3A%22Andrews%2C%20DL%20and%20Nunzi%2C%20JM%20and%20Ostendorf%2C%20A%22%7D%5D%2C%22abstractNote%22%3A%22We%20present%20theoretical%20studies%20of%20elementary%20exciton%20and%20charge%20transfer%20processes%20in%20functional%20organic%20materials%2C%20in%20view%20of%20understanding%20the%20key%20microscopic%20factors%20that%20lead%20to%20efficient%20charge%20generation%20in%20photovoltaics%20applications.%20As%20highlighted%20by%20recent%20experiments%2C%20these%20processes%20can%20be%20guided%20by%20quantum%20coherence%2C%20despite%20the%20presence%20of%20static%20and%20dynamic%20disorder.%20Our%20approach%20combines%20first-principles%20parametrized%20Hamiltonians%2C%20based%20on%20Time-Dependent%20Density%20Functional%20Theory%20%28TDDFT%29%20and%5C%2For%20high-level%20electronic%20structure%20calculations%2C%20with%20accurate%20quantum%20dynamics%20simulations%20using%20the%20Multi-Configuration%20Time-Dependent%20Hartree%20%28MCTDH%29%20method.%20This%20contribution%20specifically%20addresses%20charge%20generation%20in%20a%20novel%20class%20of%20highly%20ordered%20oligothiophene-perylene%20diimide%20type%20co-oligomer%20assemblies%2C%20highlighting%20that%20chemical%20design%20of%20donor%5C%2Facceptor%20combinations%20needs%20to%20be%20combined%20with%20a%20detailed%20understanding%20of%20the%20effects%20of%20molecular%20packing.%22%2C%22date%22%3A%222016%22%2C%22proceedingsTitle%22%3A%22NANOPHOTONICS%20VI%22%2C%22conferenceName%22%3A%22Conference%20on%20Nanophotonics%20VI%2C%20Brussels%2C%20BELGIUM%2C%2003-07%20avril%2C%202016%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1117%5C%2F12.2230314%22%2C%22ISBN%22%3A%22978-1-5106-0129-1%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1117%5C%2F12.2230314%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22CHW2VGSR%22%2C%2295EJ8IDX%22%2C%22BMA9GKQT%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222022-01-13T07%3A37%3A39Z%22%7D%7D%2C%7B%22key%22%3A%22WBXXNTDQ%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Polkehn%20et%20al.%22%2C%22parsedDate%22%3A%222016%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EM.%20Polkehn%2C%20H.%20Tamura%2C%20P.%20Eisenbrandt%2C%20S.%20Haacke%2C%20S.%20M%26%23xE9%3Bry%2C%20I.%20Burghardt%2C%20Molecular%20Packing%20Determines%20Charge%20Separation%20in%20a%20Liquid%20Crystalline%20Bisthiophene%26%23x2013%3BPerylene%20Diimide%20Donor%26%23x2013%3BAcceptor%20Material%2C%20Journal%20of%20Physical%20Chemistry%20Letters%207%20%282016%29%201327%26%23x2013%3B1334.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1021%5C%2Facs.jpclett.6b00277%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1021%5C%2Facs.jpclett.6b00277%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Molecular%20Packing%20Determines%20Charge%20Separation%20in%20a%20Liquid%20Crystalline%20Bisthiophene%5Cu2013Perylene%20Diimide%20Donor%5Cu2013Acceptor%20Material%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matthias%22%2C%22lastName%22%3A%22Polkehn%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Hiroyuki%22%2C%22lastName%22%3A%22Tamura%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Pierre%22%2C%22lastName%22%3A%22Eisenbrandt%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stefan%22%2C%22lastName%22%3A%22Haacke%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22St%5Cu00e9phane%22%2C%22lastName%22%3A%22M%5Cu00e9ry%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Irene%22%2C%22lastName%22%3A%22Burghardt%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222016%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1021%5C%2Facs.jpclett.6b00277%22%2C%22ISSN%22%3A%221948-7185%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1021%5C%2Facs.jpclett.6b00277%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22CHW2VGSR%22%2C%2295EJ8IDX%22%2C%22BMA9GKQT%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222021-06-09T13%3A02%3A18Z%22%7D%7D%2C%7B%22key%22%3A%22R3JLK4M9%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Olla%20et%20al.%22%2C%22parsedDate%22%3A%222022%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ET.%20Olla%2C%20R.%20Jabbour%2C%20A.%20Labiod%2C%20O.%20Boyron%2C%20S.%20M%26%23xE9%3Bry%2C%20B.%20Heinrich%2C%20T.%20Heiser%2C%20D.%20Jacquemin%2C%20P.%20Leveque%2C%20A.%20Lesage%2C%20N.%20Leclerc%2C%20How%20Halogenation%20Impacts%20the%20Polymer%20Backbone%20Conformation%3A%20Learning%20from%20Combination%20of%20Solid-State%20MAS%20NMR%20and%20X-Ray%20Scattering%2C%20Advanced%20Functional%20Materials%2032%20%282022%29%202204929.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fadfm.202204929%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fadfm.202204929%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22How%20Halogenation%20Impacts%20the%20Polymer%20Backbone%20Conformation%3A%20Learning%20from%20Combination%20of%20Solid-State%20MAS%20NMR%20and%20X-Ray%20Scattering%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22T%22%2C%22lastName%22%3A%22Olla%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22R%22%2C%22lastName%22%3A%22Jabbour%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22A%22%2C%22lastName%22%3A%22Labiod%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22O%22%2C%22lastName%22%3A%22Boyron%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22St%5Cu00e9phane%22%2C%22lastName%22%3A%22M%5Cu00e9ry%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Beno%5Cu0131%5Cu0302t%22%2C%22lastName%22%3A%22Heinrich%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22T%22%2C%22lastName%22%3A%22Heiser%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22D%22%2C%22lastName%22%3A%22Jacquemin%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22P%22%2C%22lastName%22%3A%22Leveque%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22A%22%2C%22lastName%22%3A%22Lesage%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22N%22%2C%22lastName%22%3A%22Leclerc%22%7D%5D%2C%22abstractNote%22%3A%22Over%20the%20past%20decade%2C%20halogenated%20semiconducting%20polymers%20have%20attracted%20considerable%20interest%20due%20to%20their%20outstanding%20optoelectronic%20properties.%20Thus%2C%20in%20most%20of%20today%27s%20organic%20photovoltaic%20devices%20benchmark%20organic%20semiconductors%20are%20halogenated%20materials%2C%20either%20electron%20donor%20polymers%20or%20non-fullerene%20acceptor%20%28NFA%29%20small%20molecules.%20However%2C%20the%20nature%20and%20position%20of%20the%20substituted%20halogen%20atoms%20in%20halogenated%20semiconducting%20polymers%20impact%2C%20through%20self-assembly%20modification%2C%20their%20optoelectronic%20properties%20in%20a%20way%20that%20is%20difficult%20to%20predict.%20Yet%2C%20the%20solid-state%20self-assembling%20of%20these%20materials%20has%20been%20shown%20to%20be%20a%20key%20parameter%20toward%20high%20charge%20transport%20properties%20and%20photovoltaic%20efficiencies.%20In%20this%20context%2C%20there%20is%20still%20a%20need%20to%20develop%20analytical%20methods%20that%20will%20enable%20an%20atomic-scale%20structural%20characterization%20of%20these%20materials%20as%20a%20function%20of%20the%20halogenation.%20In%20this%20study%2C%20the%20solid-state%20nuclear%20magnetic%20resonance%20%28NMR%29%20under%20magic%20angle%20spinning%20%28MAS%29%20is%20explored%20as%20a%20tool%20to%20investigate%20the%20local%20structure%20and%20supramolecular%20organization%20of%20a%20series%20of%20conjugated%20polymers%2C%20specially%20designed%20for%20this%20study.%20Through%20a%20comprehensive%20study%20using%20complementary%20techniques%2C%20including%20MAS-NMR%2C%20small%20and%20wide-angle%20X-ray%20scattering%2C%20and%20molecular%20modeling%20investigations%2C%20the%20molecular%20conformation%20of%20these%20polymers%20in%20relation%20to%20their%20chemical%20composition%2C%20is%20successfully%20determined.%22%2C%22date%22%3A%222022%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1002%5C%2Fadfm.202204929%22%2C%22ISSN%22%3A%221616-301X%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1002%5C%2Fadfm.202204929%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22TK3HH32E%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222022-09-27T13%3A55%3A33Z%22%7D%7D%2C%7B%22key%22%3A%22KACI3B9Q%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Olla%20et%20al.%22%2C%22parsedDate%22%3A%222019%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ET.%20Olla%2C%20O.A.%20Ibraikulov%2C%20S.%20Ferry%2C%20O.%20Boyron%2C%20S.%20M%26%23xE9%3Bry%2C%20B.%20Heinrich%2C%20T.%20Heiser%2C%20P.%20L%26%23xE9%3Bv%26%23xEA%3Bque%2C%20N.%20Leclerc%2C%20Benzothiadiazole%20Halogenation%20Impact%20in%20Conjugated%20Polymers%2C%20a%20Comprehensive%20Study%2C%20Macromolecules%2052%20%282019%29%208006%26%23x2013%3B8016.%20%3Ca%20class%3D%27zp-ItemURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1021%5C%2Facs.macromol.9b01760%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1021%5C%2Facs.macromol.9b01760%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Benzothiadiazole%20Halogenation%20Impact%20in%20Conjugated%20Polymers%2C%20a%20Comprehensive%20Study%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Th%5Cu00e9odore%22%2C%22lastName%22%3A%22Olla%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Olzhas%20A.%22%2C%22lastName%22%3A%22Ibraikulov%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22St%5Cu00e9phanie%22%2C%22lastName%22%3A%22Ferry%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Olivier%22%2C%22lastName%22%3A%22Boyron%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22St%5Cu00e9phane%22%2C%22lastName%22%3A%22M%5Cu00e9ry%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Beno%5Cu0131%5Cu0302t%22%2C%22lastName%22%3A%22Heinrich%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thomas%22%2C%22lastName%22%3A%22Heiser%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Patrick%22%2C%22lastName%22%3A%22L%5Cu00e9v%5Cu00eaque%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nicolas%22%2C%22lastName%22%3A%22Leclerc%22%7D%5D%2C%22abstractNote%22%3A%22The%20rise%20of%20halogenated%20organic%20semiconducting%20materials%20has%20led%20to%20a%20significant%20increase%20of%20organic%20photovoltaic%20power%20conversion%20efficiencies%20in%20recent%20years.%20However%2C%20the%20impact%20of%20halogen%20atoms%20on%20optoelectronic%2C%20structural%2C%20and%20photovoltaic%20properties%20is%20not%20yet%20fully%20understood.%20In%20particular%2C%20because%20of%20different%20physicochemical%20properties%2C%20the%20design%20of%20polymers%20using%20chlorine%20atoms%20instead%20of%20fluorine%20atoms%20still%20needs%20to%20be%20rationalized.%20In%20this%20paper%2C%20we%20investigate%20a%20series%20of%204%20halogenated%20D%5Cu2013A%20electron%20donor%20copolymers%2C%20by%20varying%20not%20only%20the%20number%20of%20halogen%20atoms%2C%20but%20also%20their%20nature.%20The%20in-depth%20experimental%20and%20theoretical%20study%20of%20these%20new%20polymers%2C%20using%20the%20nonhalogenated%20polymer%20as%20a%20reference%2C%20allowed%20us%20to%20rationalize%20the%20impact%20of%20these%20chemical%20modifications.%20In%20particular%2C%20we%20found%20that%20the%20structural%20properties%20and%20blending%20morphologies%20are%20mainly%20influenced%20by%20the%20nature%20of%20the%20halogen%20in%20this%20series%20of%20polymers.%20We%20have%20also%20demonstrated%20that%20a%20reasonable%20reduction%20in%20the%20number%20of%20fluorine%20atoms%20along%20the%20polymer%20backbone%20can%20be%20a%20good%20strategy%20to%20improve%20thin%20film%20processing%20conditions%20while%20keeping%20efficiencies%20at%20an%20acceptable%20level.%22%2C%22date%22%3A%222019%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1021%5C%2Facs.macromol.9b01760%22%2C%22ISSN%22%3A%220024-9297%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1021%5C%2Facs.macromol.9b01760%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22TK3HH32E%22%2C%22BMA9GKQT%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222021-06-09T13%3A03%3A17Z%22%7D%7D%2C%7B%22key%22%3A%22CQ4UTTXN%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Nestor%20et%20al.%22%2C%22parsedDate%22%3A%222017%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ES.T.%20Nestor%2C%20B.%20Heinrich%2C%20R.A.%20Sykora%2C%20X.%20Zhang%2C%20G.J.%20McManus%2C%20L.%20Douce%2C%20A.%20Mirjafari%2C%20Methimazolium-based%20ionic%20liquid%20crystals%3A%20Emergence%20of%20mesomorphic%20properties%20via%20a%20sulfur%20motif%2C%20Tetrahedron%2073%20%282017%29%205456%26%23x2013%3B5460.%20https%3A%5C%2F%5C%2Fdoi.org%5C%2F%3Ca%20class%3D%27zp-ItemURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.tet.2017.07.056%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.tet.2017.07.056%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Methimazolium-based%20ionic%20liquid%20crystals%3A%20Emergence%20of%20mesomorphic%20properties%20via%20a%20sulfur%20motif%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephen%20T.%22%2C%22lastName%22%3A%22Nestor%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Beno%5Cu00eet%22%2C%22lastName%22%3A%22Heinrich%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Richard%20A.%22%2C%22lastName%22%3A%22Sykora%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Xiaofei%22%2C%22lastName%22%3A%22Zhang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Gregory%20J.%22%2C%22lastName%22%3A%22McManus%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Laurent%22%2C%22lastName%22%3A%22Douce%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Arsalan%22%2C%22lastName%22%3A%22Mirjafari%22%7D%5D%2C%22abstractNote%22%3A%22We%20describe%20the%20proficient%20synthesis%20and%20thermotropic%20mesomorphicity%20of%20thioether%5Cu2013functionalized%20imidazolium%5Cu2013type%20ionic%20liquids%20containing%20C12%20and%20C18%20chains%20associated%20with%20Br%5Cu2212%20and%20OTf%5Cu2212%20anions.%20The%20mesomorphic%20behavior%20and%20phase%20transition%20temperatures%20were%20studied%20by%20Polarizing%20Optical%20Microscopy%20%28POM%29%2C%20Differential%20Scanning%20Calorimetry%20%28DSC%29%20and%20Small-Angle%20X-ray%20Scattering%20%28SAXS%29.%20The%20ionic%20liquids%20with%20bromide%20anion%20display%20Smectic%20A%20lamellar%20phase%20giving%20homeotropic%20and%20fan-shaped%20textures.%20The%20layered%20crystallographic%20structure%20is%20reported%20by%20Single-Crystal%20XRD.%20The%20electrochemical%20characteristics%20of%20the%20ionic%20liquids%20with%20C18%20side%20chain%20have%20been%20determined%20via%20Cyclic%20Voltammetry.%22%2C%22date%22%3A%222017%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%22https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.tet.2017.07.056%22%2C%22ISSN%22%3A%220040-4020%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.tet.2017.07.056%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22TK3HH32E%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222022-02-22T10%3A03%3A05Z%22%7D%7D%2C%7B%22key%22%3A%22588966HT%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Mouillard%20et%20al.%22%2C%22parsedDate%22%3A%222023%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EF.%20Mouillard%2C%20T.%20Fert%26%23xE9%3B%2C%20E.%20Voirin%2C%20S.%20M%26%23xE9%3Bry%2C%20P.%20Masson%2C%20A.%20Carrad%26%23xF2%3B%2C%20Use%20of%20a%20Photocleavable%20Initiator%20to%20Characterize%20Polymer%20Chains%20Grafted%20onto%20a%20Metal%20Plate%20with%20the%20Grafting-from%20Method.%2C%20Polymers%2015%20%282023%29%201265.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fpolym15051265%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3390%5C%2Fpolym15051265%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Use%20of%20a%20Photocleavable%20Initiator%20to%20Characterize%20Polymer%20Chains%20Grafted%20onto%20a%20Metal%20Plate%20with%20the%20Grafting-from%20Method.%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Flavien%22%2C%22lastName%22%3A%22Mouillard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Tom%22%2C%22lastName%22%3A%22Fert%5Cu00e9%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Emilie%22%2C%22lastName%22%3A%22Voirin%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22St%5Cu00e9phane%22%2C%22lastName%22%3A%22M%5Cu00e9ry%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Patrick%22%2C%22lastName%22%3A%22Masson%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Adele%22%2C%22lastName%22%3A%22Carrad%5Cu00f2%22%7D%5D%2C%22abstractNote%22%3A%22The%20thorough%20characterization%20of%20polymer%20chains%20grafted%20through%20a%20%5C%22grafting-from%5C%22%20process%20onto%20substrates%20based%20on%20the%20determination%20of%20number%20%28Mn%29%20and%20weight%20%28Mw%29%20average%20molar%20masses%2C%20as%20well%20as%20dispersity%20%28%5Cu0189%29%2C%20is%20quite%20challenging.%20It%20requires%20the%20cleavage%20of%20grafted%20chains%20selectively%20at%20the%20polymer-substrate%20bond%20without%20polymer%20degradation%20to%20allow%20their%20analysis%20in%20solution%20with%20steric%20exclusion%20chromatography%2C%20in%20particular.%20The%20study%20herein%20describes%20a%20technique%20for%20the%20selective%20cleavage%20of%20PMMA%20grafted%20onto%20titanium%20substrate%20%28Ti-PMMA%29%20using%20an%20anchoring%20molecule%20that%20combines%20an%20atom%20transfer%20radical%20polymerization%20%28ATRP%29%20initiator%20and%20a%20UV-cleavable%20moiety.%20This%20technique%20allows%20the%20demonstration%20of%20the%20efficiency%20of%20the%20ATRP%20of%20PMMA%20on%20titanium%20substrates%20and%20verification%20that%20the%20chains%20were%20grown%20homogeneously.%22%2C%22date%22%3A%222023%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.3390%5C%2Fpolym15051265%22%2C%22ISSN%22%3A%222073-4360%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.3390%5C%2Fpolym15051265%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22DEB5KWFS%22%2C%22MKAFAH44%22%2C%22TK3HH32E%22%2C%226739WBV7%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222023-05-31T13%3A24%3A50Z%22%7D%7D%2C%7B%22key%22%3A%228EVHA59P%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Marzuk%20et%20al.%22%2C%22parsedDate%22%3A%222018%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ES.%20Marzuk%2C%20B.%20Heinrich%2C%20P.%20Leveque%2C%20N.%20Leclerc%2C%20J.%20Khiari%2C%20S.%20M%26%23xE9%3Bry%2C%20Phthalocyanine-based%20dumbbell-shaped%20molecule%3A%20Synthesis%2C%20structure%20and%20charge%20transport%20studies%2C%20Dyes%20and%20Pigments%20154%20%282018%29%20282%26%23x2013%3B289.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.dyepig.2018.03.017%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.dyepig.2018.03.017%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Phthalocyanine-based%20dumbbell-shaped%20molecule%3A%20Synthesis%2C%20structure%20and%20charge%20transport%20studies%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22S.%22%2C%22lastName%22%3A%22Marzuk%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Beno%5Cu00eet%22%2C%22lastName%22%3A%22Heinrich%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22P.%22%2C%22lastName%22%3A%22Leveque%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22N.%22%2C%22lastName%22%3A%22Leclerc%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22J.%22%2C%22lastName%22%3A%22Khiari%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22St%5Cu00e9phane%22%2C%22lastName%22%3A%22M%5Cu00e9ry%22%7D%5D%2C%22abstractNote%22%3A%22We%20describe%20the%20synthesis%20of%20a%20fully%20conjugated%20donor-acceptor-donor%20triad%20%28ZnPc-BTD-ZnPc%29%20made%20of%20zinc%20phthalocyanine%20donor%20fragments%20%28ZnPc%29%20at%20both%20ends%20of%20a%20benzothiadiazole-based%20central%20dye%20%28BTD%29.%20The%20molecule%20exhibits%20a%20broad%20absorption%20in%20the%20whole%20visible%20range.%20The%20introduction%20of%20sterically%20demanding%20alkoxy%20chains%20to%20the%20ZnPc%20fragments%20is%20found%20to%20limit%20the%20molecular%20organization%20to%20a%20short-range%20columnar%20order%20and%20the%20charge-carrier%20mobility%20to%20moderate%20values%2C%20but%20provides%20outstanding%20solubilities%20in%20organic%20solvents.%22%2C%22date%22%3A%222018%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.dyepig.2018.03.017%22%2C%22ISSN%22%3A%220143-7208%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1016%5C%2Fj.dyepig.2018.03.017%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22TK3HH32E%22%2C%22BMA9GKQT%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222021-06-09T13%3A03%3A04Z%22%7D%7D%2C%7B%22key%22%3A%22FF6JPFEC%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Marzouk%20et%20al.%22%2C%22parsedDate%22%3A%222017%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ES.%20Marzouk%2C%20A.%20Khalfallah%2C%20B.%20Heinrich%2C%20J.E.%20Khiari%2C%20A.%20Kriaa%2C%20S.%20M%26%23xE9%3Bry%2C%20Synthesis%20and%20mesomorphic%20properties%20of%20liquid%20crystals%20containing%20a%20perfluorinated%20segment%20via%20different%20linkers%2C%20Journal%20of%20Fluorine%20Chemistry%20197%20%282017%29%2015%26%23x2013%3B23.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.jfluchem.2017.02.006%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.jfluchem.2017.02.006%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Synthesis%20and%20mesomorphic%20properties%20of%20liquid%20crystals%20containing%20a%20perfluorinated%20segment%20via%20different%20linkers%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Samir%22%2C%22lastName%22%3A%22Marzouk%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ali%22%2C%22lastName%22%3A%22Khalfallah%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Benoit%22%2C%22lastName%22%3A%22Heinrich%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jamel%20Eddine%22%2C%22lastName%22%3A%22Khiari%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Abdelkader%22%2C%22lastName%22%3A%22Kriaa%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22St%5Cu00e9phane%22%2C%22lastName%22%3A%22M%5Cu00e9ry%22%7D%5D%2C%22abstractNote%22%3A%22This%20study%20presents%20the%20synthesis%20and%20mesomorphic%20properties%20of%20a%20series%20of%20biphenyl%20benzoate%20liquid%20crystals%20carrying%20a%20perfluorinated%20segment%2C%20via%20three%20uncommon%20flexible%20linkers.%20In%20particular%2C%20a%20%28E%29-2-propenyloxy%20linker%20was%20used%20which%20was%20issued%20from%20an%20elegant%20regioselective%20dehydrohalogenation%20reaction.%20The%20remarkable%20microsegregation%20effect%20of%20the%20perfluorinated%20segment%2C%20led%20to%20the%20formation%20of%20highly%20stable%20untilted%20smectic%20mesophases.%20For%20all%20mesophases%2C%20the%20molecular%20packing%20corresponds%20to%20a%20monolayer%20arrangement%20of%20the%20mesogenic%20parts%20and%20a%20partial%20intercalation%20of%20the%20perfluorinated%20chains%2C%20measured%20by%20a%20intercalation%20ratio%20parameter%20tau%28F%29.%20Geometrical%20calculations%20based%20on%20Xrays%20data%20led%20to%20TF%20comprised%20between%201.48%20and%201.68%20depending%20of%20the%20mesophase%20and%20temperature.%20%28C%29%202017%20Elsevier%20B.V.%20All%20rights%20reserved.%22%2C%22date%22%3A%222017%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.jfluchem.2017.02.006%22%2C%22ISSN%22%3A%220022-1139%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1016%5C%2Fj.jfluchem.2017.02.006%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22TK3HH32E%22%2C%22BMA9GKQT%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222021-06-09T13%3A02%3A49Z%22%7D%7D%2C%7B%22key%22%3A%22RGXRTMNE%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Mahmoudi%20et%20al.%22%2C%22parsedDate%22%3A%222021%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EC.%20Mahmoudi%2C%20I.%20Bulut%2C%20J.%20Jing%2C%20S.%20Fall%2C%20B.%20Heinrich%2C%20S.%20M%26%23xE9%3Bry%2C%20T.%20Heiser%2C%20P.%20Leveque%2C%20E.%20Steveler%2C%20M.%20Majdoub%2C%20N.%20Leclerc%2C%20Regioisomers%20of%20Organic%20Semiconducting%20Dumbbell-Shaped%20Molecules%3A%20Synthesis%20and%20Structure-Properties%20Relationship%2C%20European%20Journal%20of%20Organic%20Chemistry%20%282021%29%203170%26%23x2013%3B3177.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fejoc.202100473%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fejoc.202100473%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Regioisomers%20of%20Organic%20Semiconducting%20Dumbbell-Shaped%20Molecules%3A%20Synthesis%20and%20Structure-Properties%20Relationship%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Chaima%22%2C%22lastName%22%3A%22Mahmoudi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ibrahim%22%2C%22lastName%22%3A%22Bulut%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jiang%22%2C%22lastName%22%3A%22Jing%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sadiara%22%2C%22lastName%22%3A%22Fall%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Benoit%22%2C%22lastName%22%3A%22Heinrich%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22St%5Cu00e9phane%22%2C%22lastName%22%3A%22M%5Cu00e9ry%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thomas%22%2C%22lastName%22%3A%22Heiser%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Patrick%22%2C%22lastName%22%3A%22Leveque%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Emilie%22%2C%22lastName%22%3A%22Steveler%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Mustapha%22%2C%22lastName%22%3A%22Majdoub%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nicolas%22%2C%22lastName%22%3A%22Leclerc%22%7D%5D%2C%22abstractNote%22%3A%22Two%20new%20dumbbell-shaped%20molecules%20based%20on%20two%20solubilizing%20and%20structuring%20triazatruxene%20%28TAT%29%20units%20linked%20by%20a%20central%20chromophore%20were%20synthesized%20and%20studied.%20The%20central%20chromophore%20was%20an%20electro-deficient%20fluorene-malononitrile%20%28FM%29%20unit%2C%20that%20can%20be%20functionalized%20symmetrically%20on%20two%20different%20positions%2C%20giving%20rise%20to%20two%20positional%20isomers%2C%20called%20TAT-pFM%20and%20TAT-mFM%2C%20when%20the%20TATs%20are%20connected%20to%20the%202%2C7-%20and%203%2C6-positions%2C%20respectively.%20The%20two%20isomers%20exhibited%20different%20electronic%20conjugation%20pathways%20that%20drastically%20affect%20their%20absorption%20properties%20and%20energy%20levels.%20Moreover%2C%20while%20TAT-pFM%20was%20organized%20in%20a%20stable%203D%20mesomorphic%20structure%20from%20room-temperature%20to%20the%20melting%20point%2C%20TAT-mFM%20remained%20crystalline%20and%20decomposed%20before%20melting.%20Finally%2C%20despite%20a%20lower%20hole%20mobility%2C%20the%20TAT-mFM%20exhibited%20the%20highest%20Power%20Conversion%20Efficiency%20%28PCE%29%20of%20about%202%20%25%20in%20organic%20solar%20cells.%20This%20higher%20PCE%20was%20attributed%20essentially%20to%20the%20pronounced%20internal%20charge%20transfer%20band%20contribution%20to%20the%20charge%20photogeneration%20observed%20in%20TAT-mFM%20solar%20cells.%22%2C%22date%22%3A%222021%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1002%5C%2Fejoc.202100473%22%2C%22ISSN%22%3A%221434-193X%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1002%5C%2Fejoc.202100473%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22TK3HH32E%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222021-08-17T12%3A37%3A10Z%22%7D%7D%2C%7B%22key%22%3A%22JK6VXUAG%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Liu%20et%20al.%22%2C%22parsedDate%22%3A%222025%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EX.%20Liu%2C%20V.%20Placide%2C%20L.%20Chu%2C%20K.M.%20Haidaraly%2C%20L.S.%20Vargas%2C%20C.%20Adachi%2C%20J.W.%20Wu%2C%20B.%20Heinrich%2C%20E.%20Lacaze%2C%20W.%20Yan%2C%20A.%20D%26%23x2019%3BAleo%2C%20F.%20Mathevet%2C%20Investigation%20and%20modulation%20of%20charge%20transport%20properties%20with%20thin%20films%20of%20an%20isoindigo-based%20donor-acceptor%20molecular%20semiconductor%2C%20Applied%20Surface%20Science%20686%20%282025%29%20162057.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.apsusc.2024.162057%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.apsusc.2024.162057%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Investigation%20and%20modulation%20of%20charge%20transport%20properties%20with%20thin%20films%20of%20an%20isoindigo-based%20donor-acceptor%20molecular%20semiconductor%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Xiao%22%2C%22lastName%22%3A%22Liu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Virginie%22%2C%22lastName%22%3A%22Placide%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Liang%22%2C%22lastName%22%3A%22Chu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Kevin%20Mall%22%2C%22lastName%22%3A%22Haidaraly%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Lydia%20Sosa%22%2C%22lastName%22%3A%22Vargas%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Chihaya%22%2C%22lastName%22%3A%22Adachi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jeong%20Weon%22%2C%22lastName%22%3A%22Wu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Benoit%22%2C%22lastName%22%3A%22Heinrich%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Emmanuelle%22%2C%22lastName%22%3A%22Lacaze%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Wensheng%22%2C%22lastName%22%3A%22Yan%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Anthony%22%2C%22lastName%22%3A%22D%27Aleo%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Fabrice%22%2C%22lastName%22%3A%22Mathevet%22%7D%5D%2C%22abstractNote%22%3A%22Charge%20mobility%20plays%20a%20crucial%20role%20in%20determining%20the%20performance%20of%20organic%20semiconducting%20devices.%20Organic%20semiconductors%20%28OSCs%29%20based%20on%20donor-acceptor%20%28D-A%29%20small%20molecules%20generally%20have%20planar%20backbones%20that%20facilitate%20charge%20transport.%20However%2C%20their%20hole%20transport%20property%20in%20thin%20film%20transistors%20%28TFTs%29%20still%20required%20to%20be%20further%20improved%2C%20and%20simultaneously%20achieving%20electron%20transport%20alongside%20hole%20transport%20remains%20a%20great%20challenge%20due%20to%20the%20presence%20of%20electron%20traps%20on%20the%20substrate%20surface.%20In%20this%20study%2C%20an%20isoindigo-based%20oligothiophene%20that%20is%20a%20D-A%20small%20molecule%2C%20was%20synthesized%20and%20employed%20as%20an%20active%20layer%20in%20TFTs.%20The%20impact%20of%20thermal%20annealing%20on%20the%20structure%2C%20morphology%20and%20charge%20transport%20properties%20of%20its%20thin%20films%20was%20investigated.%20By%20implementing%20a%20facile%20surface%20engineering%2C%20electron%20traps%20on%20the%20SiO2%20dielectric%20surface%20were%20effectively%20eliminated.%20As%20a%20result%2C%20the%20charge%20transport%20behavior%20in%20the%20TFTs%20was%20successfully%20transformed%20from%20solely%20p-type%20to%20ambipolar%20characteristics.%20This%20accomplishment%20holds%20great%20significance%20for%20the%20advancement%20of%20optoelectronic%20devices%20in%20which%20both%20p-type%20and%20n-type%20conduction%20are%20harnessed.%22%2C%22date%22%3A%222025%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.apsusc.2024.162057%22%2C%22ISSN%22%3A%220169-4332%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1016%5C%2Fj.apsusc.2024.162057%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222025-02-12T08%3A46%3A20Z%22%7D%7D%2C%7B%22key%22%3A%22KRKJXUUJ%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Liu%20et%20al.%22%2C%22parsedDate%22%3A%222016%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EL.%20Liu%2C%20P.%20Eisenbrandt%2C%20T.%20Roland%2C%20M.%20Polkehn%2C%20P.-O.%20Schwartz%2C%20K.%20Bruchlos%2C%20B.%20Omiecienski%2C%20S.%20Ludwigs%2C%20N.%20Leclerc%2C%20E.%20Zaborova%2C%20J.%20L%26%23xE9%3Bonard%2C%20S.%20M%26%23xE9%3Bry%2C%20I.%20Burghardt%2C%20S.%20Haacke%2C%20Controlling%20Charge%20Separation%20and%20Recombination%20by%20Chemical%20Design%20in%20Donor-Acceptor%20Dyads%2C%20Physical%20Chemistry%20Chemical%20Physics%2018%20%282016%29%2018536%26%23x2013%3B18548.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2FC6CP00644B%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2FC6CP00644B%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Controlling%20Charge%20Separation%20and%20Recombination%20by%20Chemical%20Design%20in%20Donor-Acceptor%20Dyads%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Li%22%2C%22lastName%22%3A%22Liu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Pierre%22%2C%22lastName%22%3A%22Eisenbrandt%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thomas%22%2C%22lastName%22%3A%22Roland%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matthias%22%2C%22lastName%22%3A%22Polkehn%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Pierre-Olivier%22%2C%22lastName%22%3A%22Schwartz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Kirsten%22%2C%22lastName%22%3A%22Bruchlos%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Beatrice%22%2C%22lastName%22%3A%22Omiecienski%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sabine%22%2C%22lastName%22%3A%22Ludwigs%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nicolas%22%2C%22lastName%22%3A%22Leclerc%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Elena%22%2C%22lastName%22%3A%22Zaborova%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22J%5Cu00e9r%5Cu00e9mie%22%2C%22lastName%22%3A%22L%5Cu00e9onard%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22St%5Cu00e9phane%22%2C%22lastName%22%3A%22M%5Cu00e9ry%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Irene%22%2C%22lastName%22%3A%22Burghardt%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stefan%22%2C%22lastName%22%3A%22Haacke%22%7D%5D%2C%22abstractNote%22%3A%22Conjugated%20donor-acceptor%20block%20co-oligomers%20that%20self-organize%20into%20D-A%20mesomorphic%20arrays%20have%20raised%20increasing%20interest%20due%20to%20their%20potential%20applications%20in%20organic%20solar%20cells.%20We%20report%20here%20a%20combined%20experimental%20and%20computational%20study%20of%20charge%20transfer%20%28CT%29%20state%20formation%20and%20recombination%20in%20isolated%20donor-spacer-acceptor%20oligomers%20based%20on%20bisthiophene-fluorene%20%28D%29%20and%20perylene%20diimide%20%28A%29%2C%20which%20have%20recently%20shown%20to%20self-organize%20to%20give%20a%20mesomorphic%20lamellar%20structure%20at%20room%20temperature.%20Using%20femtosecond%20transient%20absorption%20spectroscopy%20and%20Time-Dependent%20Density%20Functional%20Theory%20in%20combination%20with%20the%20Marcus-Jortner%20formalism%2C%20the%20observed%20increase%20of%20the%20CT%20lifetimes%20is%20rationalized%20in%20terms%20of%20a%20reduced%20electronic%20coupling%20between%20D%20and%20A%20brought%20about%20by%20the%20chemical%20design%20of%20the%20donor%20moiety.%20A%20marked%20dependence%20of%20the%20CT%20lifetime%20on%20solvent%20polarity%20is%20observed%2C%20underscoring%20the%20importance%20of%20electrostatic%20effects%20and%20those%20of%20the%20environment%20at%20large.%20The%20present%20investigation%20therefore%20calls%20for%20a%20more%20comprehensive%20design%20approach%20including%20the%20effects%20of%20molecular%20packing.%22%2C%22date%22%3A%222016%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1039%5C%2FC6CP00644B%22%2C%22ISSN%22%3A%221463-9076%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1039%5C%2FC6CP00644B%22%2C%22collections%22%3A%5B%22CHW2VGSR%22%2C%2295EJ8IDX%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222022-02-10T07%3A59%3A32Z%22%7D%7D%2C%7B%22key%22%3A%226ZGB9ZER%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Lee%20et%20al.%22%2C%22parsedDate%22%3A%222016%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EK.J.%20Lee%2C%20J.H.%20Woo%2C%20Y.%20Xiao%2C%20E.%20Kim%2C%20L.%20Mazur%2C%20D.%20Kreher%2C%20A.-J.%20Attias%2C%20K.%20Matczyszyn%2C%20M.%20Samoc%2C%20B.%20Heinrich%2C%20S.%20M%26%23xE9%3Bry%2C%20F.%20Fages%2C%20L.%20Mager%2C%20A.%20D%26%23x2019%3BAleo%2C%20J.W.%20Wu%2C%20F.%20MATHEVET%2C%20P.%20Andre%2C%20J.-C.%20Ribierre%2C%20Structure-charge%20transfer%20property%20relationship%20in%20self-assembled%20discotic%20liquid-crystalline%20donor-acceptor%20dyad%20and%20triad%20thin%20films%2C%20RSC%20Advances%206%20%282016%29%2057811%26%23x2013%3B57819.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2FC6RA08039A%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2FC6RA08039A%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Structure-charge%20transfer%20property%20relationship%20in%20self-assembled%20discotic%20liquid-crystalline%20donor-acceptor%20dyad%20and%20triad%20thin%20films%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Kwang%20Jin%22%2C%22lastName%22%3A%22Lee%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jae%20Heun%22%2C%22lastName%22%3A%22Woo%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yiming%22%2C%22lastName%22%3A%22Xiao%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Eunsun%22%2C%22lastName%22%3A%22Kim%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Leszek%22%2C%22lastName%22%3A%22Mazur%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22David%22%2C%22lastName%22%3A%22Kreher%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Andre-Jean%22%2C%22lastName%22%3A%22Attias%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Katarzyna%22%2C%22lastName%22%3A%22Matczyszyn%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Marek%22%2C%22lastName%22%3A%22Samoc%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Benoit%22%2C%22lastName%22%3A%22Heinrich%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22St%5Cu00e9phane%22%2C%22lastName%22%3A%22M%5Cu00e9ry%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Frederic%22%2C%22lastName%22%3A%22Fages%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Loic%22%2C%22lastName%22%3A%22Mager%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Anthony%22%2C%22lastName%22%3A%22D%27Aleo%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jeong%20Weon%22%2C%22lastName%22%3A%22Wu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Fabrice%22%2C%22lastName%22%3A%22MATHEVET%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Pascal%22%2C%22lastName%22%3A%22Andre%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jean-Charles%22%2C%22lastName%22%3A%22Ribierre%22%7D%5D%2C%22abstractNote%22%3A%22The%20photophysical%20properties%20of%20donor-acceptor%20%28D-A%29%20and%20donor-acceptor-donor%20%28D-A-D%29%20liquid%20crystalline%20dyad%20and%20triad%20based%20on%20two%20different%20discotic%20mesogens%20are%20examined%20in%20thin%20films%20by%20steady-state%20optical%20spectroscopy%20and%20subpicosecond%20transient%20absorption%20measurements.%20In%20these%20systems%2C%20triphenylene%20and%20perylene%20bisimide%20units%20are%20covalently%20linked%20by%20flexible%20decyloxy%20chain%28s%29%20and%20act%20as%20electron%20donor%20%28D%29%20and%20acceptor%20%28A%29%2C%20respectively.%20These%20discotic%20liquid-crystalline%20systems%20form%20well-separated%20D%20and%20A%20pi-stacked%20columnar%20structures%20in%20thin%20films.%20The%20absorption%20spectra%20of%20the%20films%20indicate%20an%20aggregation%20of%20the%20perylene%20bisimide%20and%20triphenylene%20moieties%20along%20the%20columns.%20Steady-state%20photoluminescence%20measurements%20show%20a%20strong%20fluorescence%20quenching%20that%20is%20mainly%20attributed%20to%20a%20photo-induced%20charge%20transfer%20process%20taking%20place%20between%20the%20triphenylene%20and%20perylene%20bisimide%20units.%20Subpicosecond%20transient%20absorption%20measurements%20show%20that%20the%20photoinduced%20charge%20transfer%20%28CT%29%20states%20in%20the%20dyad%20and%20triad%20films%20are%20formed%20within%200.3%20ps%20and%20recombine%20on%20a%20150-360%20ps%20time%20scale.%20In%20addition%2C%20a%20correlation%20between%20the%20dynamics%20of%20the%20charge%20recombination%20process%20and%20the%20spacing%20distances%20between%20D%20and%20A%20units%20can%20be%20established%20in%20the%20dyad%20and%20triad%20films.%20This%20study%20provides%20important%20information%20on%20the%20relationship%20between%20molecular%20packing%20and%20the%20charge%20transfer%20properties%20in%20such%20self-organized%20D%20and%20A%20columnar%20nanostructures.%22%2C%22date%22%3A%222016%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1039%5C%2FC6RA08039A%22%2C%22ISSN%22%3A%222046-2069%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1039%5C%2FC6RA08039A%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22CHW2VGSR%22%2C%22TK3HH32E%22%2C%22WWGPR7DV%22%2C%22BMA9GKQT%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222021-06-09T13%3A01%3A58Z%22%7D%7D%2C%7B%22key%22%3A%226HQY9NXA%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Labiod%20et%20al.%22%2C%22parsedDate%22%3A%222022%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EA.%20Labiod%2C%20O.A.%20Ibraikulov%2C%20S.%20Dabos-Seignon%2C%20S.%20Ferry%2C%20B.%20Heinrich%2C%20S.%20M%26%23xE9%3Bry%2C%20S.%20Fall%2C%20H.J.T.%20Nkuissi%2C%20T.%20Heiser%2C%20C.%20Cabanetos%2C%20N.%20Leclerc%2C%20P.%20Leveque%2C%20Photo-degradation%20in%20bulk%20heterojunction%20organic%20solar%20cells%20using%20a%20fullerene%20or%20a%20non-fullerene%20derivative%20electron%20acceptor%2C%20Organic%20Electronics%20107%20%282022%29%20106549.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.orgel.2022.106549%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.orgel.2022.106549%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Photo-degradation%20in%20bulk%20heterojunction%20organic%20solar%20cells%20using%20a%20fullerene%20or%20a%20non-fullerene%20derivative%20electron%20acceptor%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Amina%22%2C%22lastName%22%3A%22Labiod%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Olzhas%20A.%22%2C%22lastName%22%3A%22Ibraikulov%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sylvie%22%2C%22lastName%22%3A%22Dabos-Seignon%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephanie%22%2C%22lastName%22%3A%22Ferry%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Benoit%22%2C%22lastName%22%3A%22Heinrich%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22M%5Cu00e9ry%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sadiara%22%2C%22lastName%22%3A%22Fall%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Herve%20J.%20Tchognia%22%2C%22lastName%22%3A%22Nkuissi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thomas%22%2C%22lastName%22%3A%22Heiser%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Clement%22%2C%22lastName%22%3A%22Cabanetos%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nicolas%22%2C%22lastName%22%3A%22Leclerc%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Patrick%22%2C%22lastName%22%3A%22Leveque%22%7D%5D%2C%22abstractNote%22%3A%22The%20use%20of%20Non-Fullerene%20Acceptors%20%28NFAs%29%20in%20the%20active%20layer%20of%20organic%20solar%20cells%20%28OSCs%29%20has%20made%20it%20possible%20to%20exceed%2018%25%20conversion%20efficiency.%20However%2C%20OSCs%20still%20present%20stability%20issues%20under%20operational%20conditions%20that%20need%20to%20be%20surpassed%20for%20their%20industrialization.%20In%20this%20work%2C%20we%20investigated%20binary%20and%20ternary%20blends%20to%20examine%20their%20efficiency%20and%20their%20stability%20as%20active%20layers%20of%20OSCs.%20We%20used%20a%20fluorinated%20polymer%20%28PF2%29%20as%20an%20electron%20donor%20and%20two%20different%20electron%20acceptors%2C%20a%20fullerene%20derivative%20%28PC71BM%29%20and%20a%20NFA%20%28EH-IDTBR%29.%20We%20demonstrated%20that%20using%20EH-IDTBR%20instead%20of%20PC71BM%20leads%20to%20a%20decrease%20in%20efficiency%20attributed%20to%20the%20low%20out-of-plane%20electron%20mobility%20measured%20in%20the%20blend.%20However%2C%20using%20EH-IDTBR%20as%20single%20electron-acceptor%20significantly%20enhanced%20the%20OSCs%20stability%20under%20continuous%20illumination.%20Ternary%20blends%20were%20tested%20to%20reach%20simultaneously%20a%20high%20efficiency%20and%20a%20long-term%20stability.%20The%20best%20efficiency%5C%2Fstability%20compromise%20appeared%20to%20be%20when%20using%20EH-IDTBR%20only%20as%20electron-acceptor.%20We%20identified%20changes%20in%20the%20main%20charge-carrier%20recombination%20mechanism%20in%20photo-degraded%20devices%20from%20bimolecular%20in%20low%20EH-IDTBR%20content%20blends%20to%20trap-assisted%20in%20high%20EH-IDTBR%20ones.%20Finally%2C%20the%20blend%20morphology%20at%20a%20nanometer%20scale%20appeared%20as%20stable%20in%20high%20EH-IDTBR%20content%20blends%20while%20photo-degradation%20impacted%20significantly%20the%20morphology%20of%20the%20low%20EH-IDTBR%20content%20blend.%22%2C%22date%22%3A%222022%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.orgel.2022.106549%22%2C%22ISSN%22%3A%221566-1199%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1016%5C%2Fj.orgel.2022.106549%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22TK3HH32E%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222022-11-16T15%3A12%3A07Z%22%7D%7D%2C%7B%22key%22%3A%22ZWJ2WDF2%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22L%27Her%20et%20al.%22%2C%22parsedDate%22%3A%222020%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EM.%20L%26%23x2019%3BHer%2C%20Y.%20Atoini%2C%20J.%20Fouchet%2C%20B.%20Heinrich%2C%20N.%20Del-Giudice%2C%20E.%20Scrafton%2C%20E.%20Bordes%2C%20L.%20Karmazin%2C%20L.%20Charbonniere%2C%20L.%20De%20Cola%2C%20L.%20Douce%2C%20Luminescent%20imidazolium-naphthalene%20salts%20in%20liquid%20and%20solid%20states%20%28vol%2043%2C%20pg%2012529%2C%202019%29%2C%20New%20Journal%20of%20Chemistry%2044%20%282020%29%202669.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fd0nj90011g%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fd0nj90011g%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Luminescent%20imidazolium-naphthalene%20salts%20in%20liquid%20and%20solid%20states%20%28vol%2043%2C%20pg%2012529%2C%202019%29%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matthieu%22%2C%22lastName%22%3A%22L%27Her%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Youssef%22%2C%22lastName%22%3A%22Atoini%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Julien%22%2C%22lastName%22%3A%22Fouchet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Benoit%22%2C%22lastName%22%3A%22Heinrich%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nicolas%22%2C%22lastName%22%3A%22Del-Giudice%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Emma%22%2C%22lastName%22%3A%22Scrafton%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Emilie%22%2C%22lastName%22%3A%22Bordes%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Lydia%22%2C%22lastName%22%3A%22Karmazin%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Loic%22%2C%22lastName%22%3A%22Charbonniere%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Luisa%22%2C%22lastName%22%3A%22De%20Cola%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Laurent%22%2C%22lastName%22%3A%22Douce%22%7D%5D%2C%22abstractNote%22%3A%22corrections%22%2C%22date%22%3A%222020%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1039%5C%2Fd0nj90011g%22%2C%22ISSN%22%3A%221144-0546%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1039%5C%2Fd0nj90011g%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22TK3HH32E%22%2C%22BMA9GKQT%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222022-02-15T16%3A33%3A14Z%22%7D%7D%2C%7B%22key%22%3A%22G4URMEAC%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22L%27Her%20et%20al.%22%2C%22parsedDate%22%3A%222019%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EM.%20L%26%23x2019%3BHer%2C%20Y.%20Atoini%2C%20J.%20Fouchet%2C%20B.%20Heinrich%2C%20N.%20Del-Giudice%2C%20E.%20Scrafton%2C%20E.%20Bordes%2C%20L.%20Karmazin%2C%20L.%20Charboniere%2C%20L.%20De%20Cola%2C%20L.%20Douce%2C%20Luminescent%20imidazolium-naphthalene%20salts%20in%20liquid%20and%20solid%20states%2C%20New%20Journal%20of%20Chemistry%2043%20%282019%29%2012529%26%23x2013%3B12532.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fc9nj02972a%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fc9nj02972a%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Luminescent%20imidazolium-naphthalene%20salts%20in%20liquid%20and%20solid%20states%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matthieu%22%2C%22lastName%22%3A%22L%27Her%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Youssef%22%2C%22lastName%22%3A%22Atoini%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Julien%22%2C%22lastName%22%3A%22Fouchet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Benoit%22%2C%22lastName%22%3A%22Heinrich%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nicolas%22%2C%22lastName%22%3A%22Del-Giudice%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Emma%22%2C%22lastName%22%3A%22Scrafton%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Emilie%22%2C%22lastName%22%3A%22Bordes%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Lydia%22%2C%22lastName%22%3A%22Karmazin%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Loic%22%2C%22lastName%22%3A%22Charboniere%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Luisa%22%2C%22lastName%22%3A%22De%20Cola%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Laurent%22%2C%22lastName%22%3A%22Douce%22%7D%5D%2C%22abstractNote%22%3A%22This%20article%20describes%20highly%20luminescent%20ionic%20compounds%20in%20liquid%20and%20crystalline%20states%2C%20where%20a%20naphthalene%20moiety%20is%20conjugated%20to%20an%20imidazolium%20center%20decorated%20with%20alkyl%20chains%20of%20different%20lengths.%20We%20have%20characterized%2C%20by%20X-ray%20diffraction%20of%20a%20single%20crystal%20and%20the%20liquid%2C%20that%20these%20compounds%20are%20organized%20in%20rod-shaped%20assemblies.%20The%20formation%20of%20this%20molecular%20architecture%20is%20governed%20by%20ionic%20interactions%20that%20dominate%20those%20of%20the%20lesser%20pi-pi%20and%20van%20der%20Waals%20interactions.%20Consequently%2C%20aromatic%20naphthalene%20rings%20are%20confined%20to%20the%20environment%20of%20alkyl%20chains%20%28diluted%20in%20an%20apolar%20solvent%29%2C%20thus%20avoiding%20the%20extinction%20of%20luminescence%20by%20the%20formation%20of%20chromophore%20excimers%2C%20as%20shown%20in%20photophysical%20data.%22%2C%22date%22%3A%222019%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1039%5C%2Fc9nj02972a%22%2C%22ISSN%22%3A%221144-0546%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1039%5C%2Fc9nj02972a%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22TK3HH32E%22%2C%22BMA9GKQT%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222021-04-30T13%3A42%3A29Z%22%7D%7D%2C%7B%22key%22%3A%22MSEUEN4I%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Kumar%20et%20al.%22%2C%22parsedDate%22%3A%222020%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EK.S.%20Kumar%2C%20N.%20Del%20Giudice%2C%20B.%20Heinrich%2C%20L.%20Douce%2C%20M.%20Ruben%2C%20Bistable%20spin-crossover%20in%20a%20new%20series%20of%20%5BFe%28BPP-R%29%282%29%5D%282%2B%29%20%28BPP%3D2%2C6-bis%28pyrazol-1-yl%29pyridine%3B%20R%20%3D%20CN%29%20complexes%2C%20Dalton%20Transactions%2049%20%282020%29%2014258%26%23x2013%3B14267.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fd0dt02214d%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fd0dt02214d%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Bistable%20spin-crossover%20in%20a%20new%20series%20of%20%5BFe%28BPP-R%29%282%29%5D%282%2B%29%20%28BPP%3D2%2C6-bis%28pyrazol-1-yl%29pyridine%3B%20R%20%3D%20CN%29%20complexes%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Kuppusamy%20Senthil%22%2C%22lastName%22%3A%22Kumar%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nicolas%22%2C%22lastName%22%3A%22Del%20Giudice%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Benoit%22%2C%22lastName%22%3A%22Heinrich%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Laurent%22%2C%22lastName%22%3A%22Douce%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Mario%22%2C%22lastName%22%3A%22Ruben%22%7D%5D%2C%22abstractNote%22%3A%22Spin-crossover%20%28SCO%29%20active%20transition%20metal%20complexes%20are%20a%20class%20of%20switchable%20molecular%20materials.%20Such%20complexes%20undergo%20hysteretic%20high-spin%20%28HS%29%20to%20low-spin%20%28LS%29%20transition%2C%20and%20vice%20versa%2C%20rendering%20them%20suitable%20for%20the%20development%20of%20molecule-based%20switching%20and%20memory%20elements.%20Therefore%2C%20the%20search%20for%20SCO%20complexes%20undergoing%20abrupt%20and%20hysteretic%20SCO%2C%20that%20is%2C%20bistable%20SCO%2C%20is%20actively%20carried%20out%20by%20the%20molecular%20magnetism%20community.%20In%20this%20study%2C%20we%20report%20the%20bistable%20SCO%20characteristics%20associated%20with%20a%20new%20series%20of%20iron%28II%29%20complexes-%5BFe%28BPP-CN%29%282%29%5D%28X%29%282%29%2C%20X%20%3D%20BF4%20%281a-d%29%20or%20ClO4%20%282%29-belonging%20to%20the%20%5BFe%28BPP-R%29%282%29%5D%282%2B%29%20%28BPP%20%3D%202%2C6-bis%28pyrazol-1-yl%29pyridine%29%20family%20of%20complexes.%20Among%20the%20complexes%2C%20the%20lattice%20solvent-free%20complex%202%20showed%20a%20stable%20and%20complete%20SCO%20%28T-1%5C%2F2%20%3D%20241%20K%29%20with%20a%20thermal%20hysteresis%20width%20%28Delta%20T%29%20of%2028%20K-the%20widest%20Delta%20T%20reported%20so%20far%20for%20a%20%5BFe%28BPP-R%29%282%29%5D%28X%29%282%29%20family%20of%20complexes%2C%20showing%20abrupt%20SCO.%20The%20reproducible%20and%20bistable%20SCO%20shown%20by%20the%20relatively%20simple%20%5BFe%28BPP-CN%29%282%29%5D%28X%29%282%29%20series%20of%20molecular%20complexes%20is%20encouraging%20to%20pursue%20%5BFe%28BPP-R%29%282%29%5D%282%2B%29%20systems%20for%20the%20realization%20of%20technologically%20relevant%20SCO%20complexes.%22%2C%22date%22%3A%222020%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1039%5C%2Fd0dt02214d%22%2C%22ISSN%22%3A%221477-9226%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1039%5C%2Fd0dt02214d%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22TK3HH32E%22%2C%22BMA9GKQT%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222021-04-30T13%3A40%3A55Z%22%7D%7D%2C%7B%22key%22%3A%22X5T67SXQ%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Kraus%20et%20al.%22%2C%22parsedDate%22%3A%222014%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EI.%20Kraus%2C%20S.%20Li%2C%20A.%20Knauer%2C%20M.%20Schmutz%2C%20J.%20Faerber%2C%20C.A.%20Serra%2C%20M.%20Kohler%2C%20Continuous-Microflow%20Synthesis%20and%20Morphological%20Characterization%20of%20Multiscale%20Composite%20Materials%20Based%20on%20Polymer%20Microparticles%20and%20Inorganic%20Nanoparticles%2C%20Journal%20of%20Flow%20Chemistry%204%20%282014%29%2072%26%23x2013%3B78.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1556%5C%2FJFC-D-13-00029%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1556%5C%2FJFC-D-13-00029%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Continuous-Microflow%20Synthesis%20and%20Morphological%20Characterization%20of%20Multiscale%20Composite%20Materials%20Based%20on%20Polymer%20Microparticles%20and%20Inorganic%20Nanoparticles%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Isabelle%22%2C%22lastName%22%3A%22Kraus%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Shuning%22%2C%22lastName%22%3A%22Li%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Andrea%22%2C%22lastName%22%3A%22Knauer%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Marc%22%2C%22lastName%22%3A%22Schmutz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jacques%22%2C%22lastName%22%3A%22Faerber%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Christophe%20A.%22%2C%22lastName%22%3A%22Serra%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Michael%22%2C%22lastName%22%3A%22Kohler%22%7D%5D%2C%22abstractNote%22%3A%22This%20paper%20presents%20a%20new%20route%20to%20the%20synthesis%20of%20uniform%20and%20size-controlled%20inorganic%5C%2Forganic%20composite%20microparticles%20by%20means%20of%20microreaction%20technology.%20Au-nanoparticles%20in%20the%20range%20of%203%20to%2014%20nm%20are%20synthesized%20by%20reduction%20of%20tetrachloroauric%20acid%2C%20while%20ZnO-nanoparticles%20%28200-2000%20nm%29%20are%20synthesized%20in%20a%20continuous-flow%20two-step%20process%20using%20microtube%20arrangements%20for%20microsegmented%20flow.%20Both%20inorganic%20nanoparticles%20have%20a%20wellcontrolled%20size%20and%20narrow%20size%20distribution.%20Upon%20surface%20modification%2C%20the%20nanoparticles%20are%20then%20mixed%20on%20one%20hand%20with%20an%20acrylate-based%20monomer%20and%2C%20on%20the%20other%20hand%2C%20with%20an%20aqueous%20solution%20of%20acrylamide.%20Both%20solutions%20were%20then%20emulsified%20into%20uniform%20core-shell%20droplets%20by%20means%20of%20a%20capillary-based%20microfluidic%20device.%20Droplet%27s%20shell%20was%20hardened%20through%20UV-induced%20polymerization%2C%20whereas%20the%20core%20led%20to%20a%20hydrogel%20upon%20thermal-induced%20polymerization.%20Core-shell%20polymer%20microparticles%20%28200-300%20mu%20m%29%20with%20inorganic%20nanoparticles%20selectively%20incorporated%20into%20the%20core%20and%20the%20shell%20are%20thus%20obtained%20as%20proven%20by%20extensive%20morphological%20characterizations%20using%20electronic%20and%20optical%20microscopies.%22%2C%22date%22%3A%222014%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1556%5C%2FJFC-D-13-00029%22%2C%22ISSN%22%3A%222062-249X%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1556%5C%2FJFC-D-13-00029%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22VRM2E3H6%22%2C%22WJDNKBGA%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222021-04-30T13%3A44%3A35Z%22%7D%7D%2C%7B%22key%22%3A%22JP9JFD4V%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22K%5Cu00f6hler%20et%20al.%22%2C%22parsedDate%22%3A%222013%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EJ.M.%20K%26%23xF6%3Bhler%2C%20A.%20M%26%23xE4%3Brz%2C%20J.%20Popp%2C%20A.%20Knauer%2C%20I.%20Kraus%2C%20J.%20Faerber%2C%20C.%20Serra%2C%20Polyacrylamid%5C%2FSilver%20Composite%20Particles%20Produced%20via%20Microfluidic%20Photopolymerization%20for%20Single%20Particle-Based%20SERS%20Microsensorics%2C%20Analytical%20Chemistry%2085%20%282013%29%20313%26%23x2013%3B318.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1021%5C%2Fac302751t%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1021%5C%2Fac302751t%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Polyacrylamid%5C%2FSilver%20Composite%20Particles%20Produced%20via%20Microfluidic%20Photopolymerization%20for%20Single%20Particle-Based%20SERS%20Microsensorics%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22J.%20Michael%22%2C%22lastName%22%3A%22K%5Cu00f6hler%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Anne%22%2C%22lastName%22%3A%22M%5Cu00e4rz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22J%5Cu00fcrgen%22%2C%22lastName%22%3A%22Popp%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Andrea%22%2C%22lastName%22%3A%22Knauer%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Isabelle%22%2C%22lastName%22%3A%22Kraus%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jaques%22%2C%22lastName%22%3A%22Faerber%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Christophe%22%2C%22lastName%22%3A%22Serra%22%7D%5D%2C%22abstractNote%22%3A%22A%20micro-continuous-flow%20process%20was%20applied%20for%20the%20preparation%20of%20swellable%20polyacrylamide%20particles%20incorporating%20silver%20nanoparticles.%20These%20sensor%20particles%20are%20formed%20from%20a%20mixture%20of%20a%20colloidal%20solution%20of%20silver%20nanoparticles%20and%20monomer%20by%20a%20droplet-based%20procedure%20with%20in%20situ%20photoinitiation%20of%20polymerization%20and%20a%20subsequent%20silver%20enforcement%20in%20batch.%20The%20obtained%20polymer%20composite%20particles%20show%20a%20strong%20SERS%20effect.%20Characteristic%20Raman%20signals%20of%20aqueous%20solutions%20of%20adenine%20could%20be%20detected%20down%20to%200.1%20%5Cu03bcM%20by%20the%20use%20of%20single%20sensor%20particles.%20The%20chosen%20example%20demonstrates%20that%20the%20composite%20particles%20are%20suitable%20for%20quantitative%20microanalytical%20procedures%20with%20a%20high%20dynamic%20range%20%283%20orders%20of%20magnitude%20for%20adenine%29.%22%2C%22date%22%3A%222013%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1021%5C%2Fac302751t%22%2C%22ISSN%22%3A%220003-2700%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1021%5C%2Fac302751t%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22DEB5KWFS%22%2C%22VRM2E3H6%22%2C%22WJDNKBGA%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222021-04-30T13%3A44%3A26Z%22%7D%7D%2C%7B%22key%22%3A%22XIVVGG3G%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22K%5Cu00f6hler%20et%20al.%22%2C%22parsedDate%22%3A%222013%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EJ.M.%20K%26%23xF6%3Bhler%2C%20I.%20Kraus%2C%20J.%20Faerber%2C%20C.%20Serra%2C%20Continuous-flow%20preparation%20of%20nanoporous%20metal%5C%2Fpolymer%20composite%20particles%20by%20in%20situ%20synthesis%20of%20silver%20nanoparticles%20in%20photopolymerized%20acrylate%5C%2Fdiethylene%20glycol%20droplets%2C%20Journal%20of%20Materials%20Science%2048%20%282013%29%202158%26%23x2013%3B2166.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1007%5C%2Fs10853-012-6991-0%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1007%5C%2Fs10853-012-6991-0%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Continuous-flow%20preparation%20of%20nanoporous%20metal%5C%2Fpolymer%20composite%20particles%20by%20in%20situ%20synthesis%20of%20silver%20nanoparticles%20in%20photopolymerized%20acrylate%5C%2Fdiethylene%20glycol%20droplets%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22J.%20M.%22%2C%22lastName%22%3A%22K%5Cu00f6hler%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22I.%22%2C%22lastName%22%3A%22Kraus%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22J.%22%2C%22lastName%22%3A%22Faerber%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22C.%22%2C%22lastName%22%3A%22Serra%22%7D%5D%2C%22abstractNote%22%3A%22The%20principle%20of%20micro%20continuous-flow%20synthesis%20of%20polymer%20microparticles%20using%20a%20co-flow%20arrangement%20with%20integrated%20in%20situ%20photopolymerization%20was%20applied%20for%20the%20synthesis%20of%20nanoporous%20acrylate%20particles%20and%20for%20polymer%20composite%20microparticles%20containing%20silver%20and%20gold%5C%2Fsilver%20metal%20nanoparticles.%20The%20nano%20porosity%20is%20induced%20by%20the%20addition%20of%20diethylene%20glycol%20%28DEG%29.%20Nanoporous%20and%20composite%20polymer%20particles%20with%20sizes%20between%20about%2050%20and%20500%20mu%20m%20have%20been%20obtained%20depending%20on%20the%20diameter%20of%20the%20applied%20glass%20capillary%20and%20the%20flow%20conditions%20during%20droplet%20formation.%20DEG%20acts%20as%20a%20mediator%20for%20miscibility%20and%20allows%20the%20addition%20of%20silver%20salt%20and%20tetrachloroauric%20acid%20as%20well%20as%20ascorbic%20acid%20for%20enhancing%20the%20reduction%20of%20metal%20ions%20in%20the%20reaction%20mixture.%20The%20formation%20of%20metal%20nanoparticles%20takes%20place%20mainly%20during%20the%20UV-light-induced%20polymerization.%20The%20presence%20of%20metal%20nanoparticles%20inside%20the%20polymer%20matrix%20was%20proved%20by%20SEM%20imaging%20and%20EDX%20analysis.%22%2C%22date%22%3A%222013%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1007%5C%2Fs10853-012-6991-0%22%2C%22ISSN%22%3A%220022-2461%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1007%5C%2Fs10853-012-6991-0%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22DEB5KWFS%22%2C%22VRM2E3H6%22%2C%22WJDNKBGA%22%2C%226739WBV7%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222021-04-30T13%3A44%3A20Z%22%7D%7D%2C%7B%22key%22%3A%223JPYERYN%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Kim%20et%20al.%22%2C%22parsedDate%22%3A%222021%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EM.%20Kim%2C%20K.R.%20Choi%2C%20Y.U.%20Lee%2C%20B.%20Heinrich%2C%20S.Y.%20Ko%2C%20F.%20Mathevet%2C%20J.-C.%20Ribierre%2C%20A.%20D%26%23x2019%3BAleo%2C%20J.W.%20Wu%2C%20V.%20Placide%2C%20Natural%20Hyperbolic%20Dispersion%20with%20Anisotropic%20Epsilon-Near-Zero%20and%20Epsilon-Near-Pole%20in%20Squaraine%20Molecular%20Film%2C%20Advanced%20Optical%20Materials%20%282021%29%202101091.%20%3Ca%20class%3D%27zp-ItemURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fadom.202101091%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fadom.202101091%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Natural%20Hyperbolic%20Dispersion%20with%20Anisotropic%20Epsilon-Near-Zero%20and%20Epsilon-Near-Pole%20in%20Squaraine%20Molecular%20Film%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Minjae%22%2C%22lastName%22%3A%22Kim%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Kyu%20Ri%22%2C%22lastName%22%3A%22Choi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yeon%20Ui%22%2C%22lastName%22%3A%22Lee%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Beno%5Cu00eet%22%2C%22lastName%22%3A%22Heinrich%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Soo%20Young%22%2C%22lastName%22%3A%22Ko%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Fabrice%22%2C%22lastName%22%3A%22Mathevet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jean-Charles%22%2C%22lastName%22%3A%22Ribierre%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Anthony%22%2C%22lastName%22%3A%22D%27Aleo%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jeong%20Weon%22%2C%22lastName%22%3A%22Wu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Virginie%22%2C%22lastName%22%3A%22Placide%22%7D%5D%2C%22abstractNote%22%3A%22Abstract%20Epsilon-near-zero%20%28ENZ%29%20optical%20material%20has%20been%20employed%20in%20a%20number%20of%20novel%20linear%20and%20nonlinear%20optical%20applications%2C%20owing%20to%20the%20vanishing%20polarization%20upon%20an%20incident%20optical%20wave.%20In%20a%20uniaxial%20medium%20possessing%20hyperbolic%20energy%5Cu2013momentum%20dispersion%20of%20optical%20wave%2C%20ENZ%20can%20take%20place%20at%20ordinary%20and%20extraordinary%20permittivities.%20Organic%20thin%20films%20presenting%20a%20lamellar%20structure%20have%20been%20reported%20to%20exhibit%20a%20transverse%20negative%20hyperbolic%20dispersion%20with%20ENZ%20at%20ordinary%20permittivity.%20Here%2C%20organic%20thin%20film%20with%20ENZ%20at%20extraordinary%20permittivity%20is%20demonstrated.%20Newly%20synthesized%20polymethine%20dye%20%28i.e.%2C%20squaraine%20indolenine%20triethyleneglycol%20molecule%29%20self-organizes%20to%20form%20a%20layered%20structure%20in%20a%20pristine%20film%2C%20and%20both%20transverse%20negative%20and%20positive%20hyperbolic%20dispersions%20are%20observed%20at%20visible%20wavelengths.%20Analysis%20of%20tens-nanometer-thick%20pristine%20film%20shows%20that%20both%20ENZ%20and%20epsilon-near-pole%20%28ENP%29%20occur%20at%20longitudinal%20as%20well%20as%20transverse%20component%20of%20dielectric%20permittivity.%20Optical%20characterization%20of%20squaraine%20pristine%20film%20is%20presented%2C%20and%20the%20importance%20of%20transverse%20positive%20hyperbolic%20dispersion%20in%20such%20monolithic%20thin%20film%20is%20discussed.%22%2C%22date%22%3A%222021%22%2C%22language%22%3A%22%22%2C%22DOI%22%3A%2210.1002%5C%2Fadom.202101091%22%2C%22ISSN%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fadom.202101091%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22TK3HH32E%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222022-01-24T08%3A41%3A22Z%22%7D%7D%2C%7B%22key%22%3A%22WSPFDSQJ%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Khan%20et%20al.%22%2C%22parsedDate%22%3A%222015%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EI.U.%20Khan%2C%20C.A.%20Serra%2C%20N.%20Anton%2C%20M.%20Er-Rafik%2C%20C.%20Blanck%2C%20M.%20Schmutz%2C%20I.%20Kraus%2C%20N.%20Messaddeq%2C%20C.%20Sutter%2C%20H.%20Anton%2C%20A.S.%20Klymchenko%2C%20T.F.%20Vandamme%2C%20Microfluidic%20conceived%20Trojan%20microcarriers%20for%20oral%20delivery%20of%20nanoparticles%2C%20International%20Journal%20of%20Pharmaceutics%20493%20%282015%29%207%26%23x2013%3B15.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.ijpharm.2015.06.028%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.ijpharm.2015.06.028%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Microfluidic%20conceived%20Trojan%20microcarriers%20for%20oral%20delivery%20of%20nanoparticles%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ikram%20Ullah%22%2C%22lastName%22%3A%22Khan%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Christophe%20A.%22%2C%22lastName%22%3A%22Serra%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nicolas%22%2C%22lastName%22%3A%22Anton%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Meriem%22%2C%22lastName%22%3A%22Er-Rafik%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22C.%22%2C%22lastName%22%3A%22Blanck%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Marc%22%2C%22lastName%22%3A%22Schmutz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Isabelle%22%2C%22lastName%22%3A%22Kraus%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nadia%22%2C%22lastName%22%3A%22Messaddeq%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Christophe%22%2C%22lastName%22%3A%22Sutter%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Halina%22%2C%22lastName%22%3A%22Anton%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Andrey%20S.%22%2C%22lastName%22%3A%22Klymchenko%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thierry%20F.%22%2C%22lastName%22%3A%22Vandamme%22%7D%5D%2C%22abstractNote%22%3A%22In%20this%20study%2C%20we%20report%20on%20a%20novel%20method%20for%20the%20synthesis%20of%20poly%28acrylamide%29%20Trojan%20microparticles%20containing%20ketoprofen%20loaded%20poly%28ethyl%20acrylate%29%20or%20poly%28methyl%20acrylate%29%20nanoparticles.%20To%20develop%20these%20composite%20particles%2C%20a%20polymerizable%20nanoemulsion%20was%20used%20as%20a%20template.%20This%20nanoemulsion%20was%20obtained%20in%20an%20elongational-flow%20micromixer%20%28mu%20RMX%29%20which%20was%20linked%20to%20a%20capillary-based%20microfluidic%20device%20for%20its%20emulsification%20into%20micron%20range%20droplets.%20Downstream%2C%20the%20microdroplets%20were%20hardened%20into%20Trojan%20particles%20in%20the%20size%20range%20of%20213-308%20mu%20m%20by%20UV%20initiated%20free%20radical%20polymerization.%20The%20nanoemulsion%20size%20varied%20from%2098%20-132%20nm%20upon%20changes%20in%20surfactant%20concentration%20and%20number%20of%20operating%20cycles%20in%20mu%20RMX.%20SEM%20and%20confocal%20microscopy%20confirmed%20the%20Trojan%20morphology.%20Under%20SEM%20it%20was%20observed%20that%20the%20polymerization%20reduced%20the%20size%20of%20the%20nanoemulsion%20down%20to%2020-32%20nm%20for%20poly%28ethyl%20acrylate%29%20and%2010-15%20nm%20for%20poly%28methyl%20acrylate%29%20nanoparticles.%20This%20shrinkage%20was%20confirmed%20by%20cryo-TEM%20studies.%20We%20further%20showed%20that%20Trojan%20microparticles%20released%20embedded%20nanoparticles%20on%20contact%20with%20suitable%20media%20as%20confirmed%20by%20transmission%20electron%20microscopy.%20In%20a%20USP%20phosphate%20buffer%20solution%20of%20pH%206.8%2C%20Trojan%20microparticles%20containing%20poly%28ethyl%20acrylate%29%20nanoparticles%20released%2035%25%20of%20encapsulated%20ketoprofen%20over%2024%20h.%20The%20low%20release%20of%20the%20drug%20was%20attributed%20to%20the%20overall%20low%20concentration%20of%20nanoparticles%20and%20attachment%20of%20some%20of%20nanoparticles%20to%20the%20poly%28acrylamide%29%20matrix.%20Thus%2C%20this%20novel%20method%20has%20shown%20possibility%20to%20develop%20Trojan%20particles%20convieniently%20with%20potential%20to%20deliver%20nanoparticles%20in%20the%20gastrointestinal%20tract.%20%28C%29%202015%20Elsevier%20B.V.%20All%20rights%20reserved.%22%2C%22date%22%3A%222015%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.ijpharm.2015.06.028%22%2C%22ISSN%22%3A%220378-5173%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1016%5C%2Fj.ijpharm.2015.06.028%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22VRM2E3H6%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222021-04-30T13%3A44%3A10Z%22%7D%7D%2C%7B%22key%22%3A%22JVBIXX3W%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Khan%20et%20al.%22%2C%22parsedDate%22%3A%222014%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EI.U.%20Khan%2C%20C.A.%20Serra%2C%20N.%20Anton%2C%20X.%20Li%2C%20R.%20Akasov%2C%20N.%20Messaddeq%2C%20I.%20Kraus%2C%20T.F.%20Vandamme%2C%20Microfluidic%20conceived%20drug%20loaded%20Janus%20particles%20in%20side-by-side%20capillaries%20device%2C%20International%20Journal%20of%20Pharmaceutics%20473%20%282014%29%20239%26%23x2013%3B249.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.ijpharm.2014.06.035%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.ijpharm.2014.06.035%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Microfluidic%20conceived%20drug%20loaded%20Janus%20particles%20in%20side-by-side%20capillaries%20device%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ikram%20Ullah%22%2C%22lastName%22%3A%22Khan%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Christophe%20A.%22%2C%22lastName%22%3A%22Serra%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nicolas%22%2C%22lastName%22%3A%22Anton%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Xiang%22%2C%22lastName%22%3A%22Li%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Roman%22%2C%22lastName%22%3A%22Akasov%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nadia%22%2C%22lastName%22%3A%22Messaddeq%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Isabelle%22%2C%22lastName%22%3A%22Kraus%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thierry%20F.%22%2C%22lastName%22%3A%22Vandamme%22%7D%5D%2C%22abstractNote%22%3A%22A%20side-by-side%20capillaries%20microfluidic%20device%20was%20developed%20to%20fabricate%20drug%20loaded%20poly%28acrylamide%29%5C%2Fpoly%28methyl%20acrylate%29%20Janus%20particles%20in%20the%20range%20of%2059-240%20mu%20m%20by%20UV-assisted%20free%20radical%20polymerization.%20This%20system%20was%20characterized%20in%20terms%20of%20continuous%20and%20dispersed%20phases%20flow%20rates%20%28Q%28c%29%5C%2FQ%28d%29%29%2C%20monomer%20composition%20of%20the%20two%20compartments%2C%20surfactant%20nature%20and%20concentration%2C%20outlet%20tube%20diameter%20and%20UV%20intensity.%20These%20factors%20were%20adequately%20controlled%20to%20get%20different%20particle%20shapes%20ranging%20from%20core-shell%20to%20bi-compartmental%20particles.%20For%20the%20latter%2C%20a%20low%20surfactant%20concentration%20%280.75%20wt.%25%29%20was%20necessary%20when%20the%20two%20dispersed%20phases%20were%20pumped%20at%20equal%20flow%20rate%2C%20while%20at%20high%20surfactant%20concentration%2C%20dispersed%20phases%20flow%20rates%20have%20to%20be%20changed.%20FTIR%20analysis%20suggested%20complete%20polymerization%20of%20monomers%20and%20cytotoxicity%20test%20showed%20these%20particles%20were%20biocompatible%20having%20LD%2050%20of%209%20mg%5C%2FmL.%20Both%20ketoprofen%20and%20sodium%20fluorescein%20were%20released%20in%20sustained%20release%20manner%20at%20pH%206.8%20by%20following%20a%20diffusion%20type%20release%20mechanism.%20Drug%20release%20was%20faster%20for%20bigger%20particles%20and%20found%20to%20result%20from%20the%20irregular%20distribution%20of%20the%20two%20phases%20and%20indentation%20on%20bigger%20particles%20as%20revealed%20by%20SEM%20analysis.%20In%20comparison%2C%20sodium%20fluorescein%20release%20was%20slower%20which%20was%20attributed%20to%20low%20encapsulation%20but%20could%20be%20modified%20by%20decreasing%20crosslinker%20concentration.%20%28C%29%202014%20Elsevier%20B.V.%20All%20rights%20reserved.%22%2C%22date%22%3A%222014%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.ijpharm.2014.06.035%22%2C%22ISSN%22%3A%220378-5173%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1016%5C%2Fj.ijpharm.2014.06.035%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22VRM2E3H6%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222021-04-30T13%3A43%3A58Z%22%7D%7D%2C%7B%22key%22%3A%22NDKS4EQ8%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Kamatham%20et%20al.%22%2C%22parsedDate%22%3A%222021%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EN.%20Kamatham%2C%20O.A.%20Ibraikulov%2C%20P.%20Durand%2C%20J.%20Wang%2C%20O.%20Boyron%2C%20B.%20Heinrich%2C%20T.%20Heiser%2C%20P.%20Leveque%2C%20N.%20Leclerc%2C%20S.%20M%26%23xE9%3Bry%2C%20On%20the%20Impact%20of%20Linear%20Siloxanated%20Side%20Chains%20on%20the%20Molecular%20Self-Assembling%20and%20Charge%20Transport%20Properties%20of%20Conjugated%20Polymers%2C%20Advanced%20Functional%20Materials%2031%20%282021%29%202007734.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fadfm.202007734%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fadfm.202007734%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22On%20the%20Impact%20of%20Linear%20Siloxanated%20Side%20Chains%20on%20the%20Molecular%20Self-Assembling%20and%20Charge%20Transport%20Properties%20of%20Conjugated%20Polymers%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Narayanaswamy%22%2C%22lastName%22%3A%22Kamatham%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Olzhas%20A.%22%2C%22lastName%22%3A%22Ibraikulov%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Pablo%22%2C%22lastName%22%3A%22Durand%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jing%22%2C%22lastName%22%3A%22Wang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Olivier%22%2C%22lastName%22%3A%22Boyron%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Benoit%22%2C%22lastName%22%3A%22Heinrich%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thomas%22%2C%22lastName%22%3A%22Heiser%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Patrick%22%2C%22lastName%22%3A%22Leveque%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nicolas%22%2C%22lastName%22%3A%22Leclerc%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22St%5Cu00e9phane%22%2C%22lastName%22%3A%22M%5Cu00e9ry%22%7D%5D%2C%22abstractNote%22%3A%22Herein%20reported%20is%20the%20impact%20of%20the%20functionalization%20of%20four%20different%20semiconducting%20polymer%20structures%20by%20a%20linear%20siloxane-terminated%20side-chains.%20The%20latter%20is%20tetrasiloxane%20%28Si-4%29%20or%20trisiloxane%20%28Si-3%29%20chains%2C%20substituted%20at%20their%20extremity%20to%20a%20pentylene%20linker.%20The%20polymer%20structure%20is%20based%20on%205%2C6-difluorobenzothiadiazole%20comonomer%20%28PF2%29%2C%20a%20diketopyrrolopyrrole%20unit%20%28PDPP-TT%29%2C%20a%20naphtalediimide%20unit%20%28PNDI-T-2%29%2C%20and%20a%20poly%5Bbis%28thiophen-2-yl%29thieno%5B3%2C2%2Cb%5Dthiophene%20%28PBTTT%29.%20The%20properties%20of%20these%20siloxane-functionalized%20polymers%20are%20scrutinized%20and%20compared%20with%20the%20ones%20of%20their%20alkyl-substituted%20polymer%20analogues.%20The%20impact%20of%20the%20alkyl-to-siloxane%20chain%20substitution%20clearly%20depends%20on%20the%20molecular%20section%20of%20the%20side%20chains.%20When%20a%20branched%202-octyldodecyl%20chain%20%28C-20%29%20is%20replaced%20by%20a%20Si-4%20chain%20of%20same%20molecular%20section%2C%20the%20greatest%20impact%20is%20the%20strong%20increase%20of%20the%20pi-stacking%20overlap%20of%20the%20polymer%20backbones.%20This%20effect%20leads%20to%20a%20significative%20enhancement%20of%20the%20charge%20mobility%20values%20of%20the%20polymers.%20As%20in-plane%20and%20out-of-plane%20mobility%20are%20increased%20simultaneously%2C%20this%20pi-overlap%20enhancement%20effect%20happens%20to%20be%20preponderant%20over%20the%20polymer%20orientation%20variations.%20When%20a%20linear%20tetradecyl%20chain%20%28C-14%29%20is%20replaced%20by%20a%20linear%20Si-3%20chain%20of%20twice%20larger%20molecular%20section%2C%20the%20polymer%20structure%20is%20profoundly%20affected.%20While%20PBTTT-C-14%20is%20crystalline%20and%20purely%20edge-on%2C%20PBTTT-Si-3%20is%20mesomorphic%20and%20shows%20a%20mixed%20face-on%5C%2Fedge-on%20orientation.%22%2C%22date%22%3A%222021%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1002%5C%2Fadfm.202007734%22%2C%22ISSN%22%3A%221616-301X%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1002%5C%2Fadfm.202007734%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22TK3HH32E%22%2C%22BMA9GKQT%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222021-06-09T13%3A03%3A42Z%22%7D%7D%2C%7B%22key%22%3A%22UV4YXBXW%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Jing%20et%20al.%22%2C%22parsedDate%22%3A%222022%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EJ.%20Jing%2C%20E.%20Steveler%2C%20N.%20Leclerc%2C%20A.%20D%26%23x2019%3BAleo%2C%20B.%20Heinrich%2C%20W.%20Uhring%2C%20T.%20Heiser%2C%20Exciton%20lifetime%20in%20donor-acceptor-donor%20planar%20dumbbell-shaped%20triazatruxene-thienopyrroledione%20derivatives%2C%20in%3A%20S.%20Reineke%2C%20K.%20Vandewal%2C%20W.%20Maes%20%28Eds.%29%2C%20ORGANIC%20ELECTRONICS%20AND%20PHOTONICS%3A%20FUNDAMENTALS%20AND%20DEVICES%20III%2C%20SPIE-INT%20SOC%20OPTICAL%20ENGINEERING%2C%202022.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1117%5C%2F12.2615963%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1117%5C%2F12.2615963%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22conferencePaper%22%2C%22title%22%3A%22Exciton%20lifetime%20in%20donor-acceptor-donor%20planar%20dumbbell-shaped%20triazatruxene-thienopyrroledione%20derivatives%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jiang%22%2C%22lastName%22%3A%22Jing%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Emilie%22%2C%22lastName%22%3A%22Steveler%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nicolas%22%2C%22lastName%22%3A%22Leclerc%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Anthony%22%2C%22lastName%22%3A%22D%27Aleo%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Benoit%22%2C%22lastName%22%3A%22Heinrich%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Wilfried%22%2C%22lastName%22%3A%22Uhring%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thomas%22%2C%22lastName%22%3A%22Heiser%22%7D%2C%7B%22creatorType%22%3A%22editor%22%2C%22firstName%22%3A%22S%22%2C%22lastName%22%3A%22Reineke%22%7D%2C%7B%22creatorType%22%3A%22editor%22%2C%22firstName%22%3A%22K%22%2C%22lastName%22%3A%22Vandewal%22%7D%2C%7B%22creatorType%22%3A%22editor%22%2C%22firstName%22%3A%22W%22%2C%22lastName%22%3A%22Maes%22%7D%5D%2C%22abstractNote%22%3A%22Organic%20semiconductor%20materials%20such%20as%20planar%20conjugated%20small%20molecules%20are%20of%20great%20interest%20to%20the%20photovoltaic%20community.%20In%20thin%20films%2C%20the%20exciton%20and%20charge%20carrier%20dynamics%2C%20which%20are%20crucial%20to%20photovoltaic%20device%20operation%2C%20depend%20in%20a%20non-trivial%20way%20on%20the%20organic%20molecular%20structure%20and%20on%20the%20molecular%20organization%20in%20the%20solid%20state.%20Recently%2C%20the%20exciton%20diffusion%20has%20been%20found%20to%20strongly%20depend%20on%20the%20crystalline%20order%20of%20the%20organic%20thin%20films.%20This%20work%20presents%20the%20study%20of%20the%20exciton%20lifetime%20in%20an%20innovative%20class%20of%20molecular%20semiconductors%20able%20to%20present%20different%20crystalline%20order.%20This%20family%20of%20molecules%20has%20a%20%5Cu201cdumbbell-shaped%5Cu201d%20structure%20based%20on%20triazatruxene%20units%20that%20act%20as%20a%20pi-stacking%20platform.%20Such%20molecules%20with%20different%20side-chains%20have%20been%20found%20to%20self-assemble%20into%20various%20crystalline%20and%20liquid%20crystalline%20phases.%20We%20have%20studied%20the%20steady-state%20photoluminescence%20and%20the%20exciton%20lifetime%20for%20several%20triazatruxene-based%20derivatives%20with%20different%20side-chains%2C%20in%20solution%20and%20in%20thin%20films%20for%20different%20solid%20state%20phases.%20In%20solution%2C%20the%20fluorescence%20lifetime%20corresponds%20to%20the%20reference%20value%20that%20can%20be%20obtained%20without%20intermolecular%20interaction.%20In%20thin%20films%2C%20we%20measured%20the%20exciton%20lifetime%20for%20different%20molecular%20structures%20in%20order%20to%20correlate%20the%20exciton%20dynamics%20with%20the%20molecular%20stacking.%20The%20results%20reveal%20a%20significant%20increase%20in%20the%20exciton%20lifetime%20with%20the%20enhancement%20of%20the%20structural%20order.%22%2C%22date%22%3A%222022%22%2C%22proceedingsTitle%22%3A%22ORGANIC%20ELECTRONICS%20AND%20PHOTONICS%3A%20FUNDAMENTALS%20AND%20DEVICES%20III%22%2C%22conferenceName%22%3A%22Conference%20on%20Organic%20Electronics%20and%20Photonics%20-%20Fundamentals%20and%20Devices%20III%2C%20ELECTR%20NETWORK%2C%20APR%2003-MAY%2020%2C%202022%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1117%5C%2F12.2615963%22%2C%22ISBN%22%3A%22978-1-5106-5175-3%20978-1-5106-5174-6%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1117%5C%2F12.2615963%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22TK3HH32E%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222024-06-03T09%3A40%3A21Z%22%7D%7D%2C%7B%22key%22%3A%22SKNTKS3U%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Jing%20et%20al.%22%2C%22parsedDate%22%3A%222021%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EJ.%20Jing%2C%20B.%20Heinrich%2C%20A.%20Prel%2C%20E.%20Steveler%2C%20T.%20Han%2C%20I.%20Bulut%2C%20S.%20M%26%23xE9%3Bry%2C%20Y.%20Leroy%2C%20N.%20Leclerc%2C%20P.%20Leveque%2C%20M.%20Rosenthal%2C%20D.A.%20Ivanov%2C%20T.%20Heiser%2C%20Efficient%203D%20charge%20transport%20in%20planar%20triazatruxene-based%20dumbbell-shaped%20molecules%20forming%20a%20bridged%20columnar%20phase%2C%20Journal%20of%20Materials%20Chemistry%20A%209%20%282021%29%2024315%26%23x2013%3B24324.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fd1ta06300f%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fd1ta06300f%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Efficient%203D%20charge%20transport%20in%20planar%20triazatruxene-based%20dumbbell-shaped%20molecules%20forming%20a%20bridged%20columnar%20phase%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jiang%22%2C%22lastName%22%3A%22Jing%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Benoit%22%2C%22lastName%22%3A%22Heinrich%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Alexis%22%2C%22lastName%22%3A%22Prel%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Emilie%22%2C%22lastName%22%3A%22Steveler%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Tianyan%22%2C%22lastName%22%3A%22Han%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ibrahim%22%2C%22lastName%22%3A%22Bulut%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22M%5Cu00e9ry%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yann%22%2C%22lastName%22%3A%22Leroy%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nicolas%22%2C%22lastName%22%3A%22Leclerc%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Patrick%22%2C%22lastName%22%3A%22Leveque%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Martin%22%2C%22lastName%22%3A%22Rosenthal%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Dimitri%20A.%22%2C%22lastName%22%3A%22Ivanov%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thomas%22%2C%22lastName%22%3A%22Heiser%22%7D%5D%2C%22abstractNote%22%3A%22Planar%20conjugated%20molecular%20backbones%20are%20essential%20for%20achieving%20high%20charge%20carrier%20mobilities%20along%20molecular%20pi-stacking%20directions%20but%20are%20often%20concomitant%20with%20poor%20charge%20transport%20in%20other%20directions.%20This%20is%20particularly%20the%20case%20for%20molecules%20that%20are%20functionalized%20with%20alkyl%20chains%2C%20which%20ensure%20good%20processability%20in%20solution%20but%20introduce%20detrimental%20insulating%20regions.%20Here%2C%20we%20show%20that%20soluble%20planar%20dumbbell-shaped%20molecules%2C%20composed%20of%20two%20triazatruxene%20%28TAT%29%20units%20covalently%20bonded%20to%20a%20central%20thiophene-thienopyrroledione-thiophene%20%28TPD%29%20segment%20self-assemble%20into%20an%20original%20structure%20that%20allows%20efficient%203D%20charge%20transport.%20Grazing-incidence%20wide-angle%20X-ray%20scattering%20investigations%20as%20well%20as%20micro-focus%20X-ray%20experiments%20on%20single%20crystals%20reveal%20that%20the%20TAT%20derivatives%20form%20a%20columnar-nematic%20mesophase%20in%20which%20columns%20of%20stacked%20TAT%20units%20spaced%20by%20molten%20chains%20are%20interconnected%20by%20TPD%20bridges.%20Upon%20annealing%2C%20a%20crystalline%20phase%2C%20stemming%20from%20the%20parent%20hexagonal%20mesophase%2C%20is%20obtained%20with%20the%20molecular%20stacking%20direction%20lying%20in-plane.%20Transport%20measurements%20in%20the%20crystalline%20phase%20reveal%20an%20unusually%20high%20out-of-plane%20hole%20mobility%20of%200.17%20cm%282%29%20V-1%20s%28-1%29%20and%20a%20lower%20limit%20for%20the%20in-plane%20mobility%20of%200.05%20cm%282%29%20V-1%20s%28-1%29.%20The%20results%20suggest%20that%20the%20TPD%20segments%20bridging%20neighboring%20stacks%20of%20TAT%20columns%20are%20responsible%20for%20efficient%20hole%20transport%20in%203D.%22%2C%22date%22%3A%222021%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1039%5C%2Fd1ta06300f%22%2C%22ISSN%22%3A%222050-7488%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1039%5C%2Fd1ta06300f%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22TK3HH32E%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222022-01-06T10%3A24%3A20Z%22%7D%7D%2C%7B%22key%22%3A%227JWKR9D2%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Ibraikulov%20et%20al.%22%2C%22parsedDate%22%3A%222019%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EOlzhas.A.%20Ibraikulov%2C%20J.%20Wang%2C%20N.%20Kamatham%2C%20B.%20Heinrich%2C%20S.%20M%26%23xE9%3Bry%2C%20M.%20Kohlst%26%23xE4%3Bdt%2C%20U.%20W%26%23xFC%3Brfel%2C%20S.%20Ferry%2C%20N.%20Leclerc%2C%20T.%20Heiser%2C%20P.%20L%26%23xE9%3Bv%26%23xEA%3Bque%2C%20ITO-Free%20Organic%20Photovoltaic%20Modules%20Based%20on%20Fluorinated%20Polymers%20Deposited%20from%20Non-Halogenated%20Solution%3A%20A%20Major%20Step%20Toward%20Large-Scale%20Module%20Production%2C%20Solar%20RRL%203%20%282019%29%201900273.%20%3Ca%20class%3D%27zp-ItemURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fsolr.201900273%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fsolr.201900273%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22ITO-Free%20Organic%20Photovoltaic%20Modules%20Based%20on%20Fluorinated%20Polymers%20Deposited%20from%20Non-Halogenated%20Solution%3A%20A%20Major%20Step%20Toward%20Large-Scale%20Module%20Production%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Olzhas.%20A.%22%2C%22lastName%22%3A%22Ibraikulov%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jing%22%2C%22lastName%22%3A%22Wang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Narayanaswamy%22%2C%22lastName%22%3A%22Kamatham%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Beno%5Cu00eet%22%2C%22lastName%22%3A%22Heinrich%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22St%5Cu00e9phane%22%2C%22lastName%22%3A%22M%5Cu00e9ry%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Markus%22%2C%22lastName%22%3A%22Kohlst%5Cu00e4dt%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Uli%22%2C%22lastName%22%3A%22W%5Cu00fcrfel%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22St%5Cu00e9phanie%22%2C%22lastName%22%3A%22Ferry%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nicolas%22%2C%22lastName%22%3A%22Leclerc%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thomas%22%2C%22lastName%22%3A%22Heiser%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Patrick%22%2C%22lastName%22%3A%22L%5Cu00e9v%5Cu00eaque%22%7D%5D%2C%22abstractNote%22%3A%22Several%20bottlenecks%20need%20to%20be%20passed%20to%20reach%20market%20readiness%20for%20organic%20photovoltaic%20modules.%20To%20avoid%20scarce%20and%20costly%20materials%20such%20as%20indium%20is%20an%20important%20technological%20issue.%20To%20be%20able%20to%20process%20the%20active%20layer%20from%20solutions%20prepared%20from%20non-halogenated%20solvents%20and%20harmless%20additives%20is%20also%20a%20crucial%20step.%20Herein%2C%20a%20fluorinated%20polymer%20is%20used%20as%20an%20electron-donor%20material%20and%20PC71BM%20as%20an%20electron-accepting%20material%20in%20a%20bulk-heterojunction%20configuration%2C%20to%20demonstrate%20that%20indium-tin-oxide%20%28ITO%29-free%20modules%20with%20an%20active%20area%20greater%20than%2060%20cm2%20and%20processed%20from%20non-halogenated%20solvents%20and%20harmless%20additives%20can%20reach%20a%20high%20power%20conversion%20efficiency%20of%20above%206%25.%22%2C%22date%22%3A%222019%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1002%5C%2Fsolr.201900273%22%2C%22ISSN%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fsolr.201900273%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22TK3HH32E%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222022-01-24T08%3A29%3A52Z%22%7D%7D%2C%7B%22key%22%3A%22K444H2N3%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Ibraikulov%20et%20al.%22%2C%22parsedDate%22%3A%222018%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EO.A.%20Ibraikulov%2C%20C.%20Ngov%2C%20P.%20Chavez%2C%20I.%20Bulut%2C%20B.%20Heinrich%2C%20O.%20Boyron%2C%20K.L.%20Gerasimov%2C%20D.A.%20Ivanov%2C%20S.%20Swaraj%2C%20S.%20M%26%23xE9%3Bry%2C%20N.%20Leclerc%2C%20P.%20Leveque%2C%20T.%20Heiser%2C%20Face-on%20orientation%20of%20fluorinated%20polymers%20conveyed%20by%20long%20alkyl%20chains%3A%20a%20prerequisite%20for%20high%20photovoltaic%20performances%2C%20Journal%20of%20Materials%20Chemistry%20A%206%20%282018%29%2012038%26%23x2013%3B12045.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fc8ta04127j%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fc8ta04127j%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Face-on%20orientation%20of%20fluorinated%20polymers%20conveyed%20by%20long%20alkyl%20chains%3A%20a%20prerequisite%20for%20high%20photovoltaic%20performances%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Olzhas%20A.%22%2C%22lastName%22%3A%22Ibraikulov%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Chheng%22%2C%22lastName%22%3A%22Ngov%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Patricia%22%2C%22lastName%22%3A%22Chavez%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ibrahim%22%2C%22lastName%22%3A%22Bulut%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Benoit%22%2C%22lastName%22%3A%22Heinrich%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Olivier%22%2C%22lastName%22%3A%22Boyron%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Kirill%20L.%22%2C%22lastName%22%3A%22Gerasimov%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Dimitri%20A.%22%2C%22lastName%22%3A%22Ivanov%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sufal%22%2C%22lastName%22%3A%22Swaraj%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22St%5Cu00e9phane%22%2C%22lastName%22%3A%22M%5Cu00e9ry%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nicolas%22%2C%22lastName%22%3A%22Leclerc%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Patrick%22%2C%22lastName%22%3A%22Leveque%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Thomas%22%2C%22lastName%22%3A%22Heiser%22%7D%5D%2C%22abstractNote%22%3A%22The%20recently%20reported%20high%20power%20conversion%20efficiencies%20achieved%20in%20fluorinated%20polymer%3Afullerene%20solar%20cells%20have%20been%20accounted%20for%20by%20the%20presence%20of%20face-on%20oriented%20polymer%20backbones%20that%20enable%20charge%20transport%20towards%20the%20collecting%20electrodes.%20In%20this%20work%2C%20we%20demonstrate%20that%2C%20in%20contrast%20to%20the%20results%20of%20a%20number%20of%20reports%2C%20the%20face-on%20polymer%20orientation%20is%20due%20to%20the%20bulky%20side%20chains%2C%20rather%20than%20to%20aggregation%20in%20solution.%20This%20conclusion%20is%20supported%20by%20a%20comparative%20study%20of%20polymers%20having%20similar%20conjugated%20backbones%20but%20different%20number%20of%20fluorine%20atoms%20and%20different%20number%20and%20type%20of%20alkyl%20side%20chains.%20While%20the%20latter%20are%20primarily%20introduced%20to%20tune%20polymer%20solubility%2C%20the%20present%20in-depth%20thin-film%20morphology%20investigation%20shows%20that%20increasing%20the%20chain%20bulkiness%20favors%20formation%20of%20crystalline%20lamellae%20with%20face-on%20oriented%20backbones%2C%20independently%20of%20the%20degree%20of%20fluorination.%20By%20contrast%2C%20introduction%20of%20fluorine%20atoms%20is%20found%20to%20substantially%20enhance%20the%20-stacking%20interactions%20that%20remain%20invariably%20strong%20upon%20blending%20of%20the%20polymer%20with%20fullerene.%20Our%20results%20demonstrate%20that%2C%20for%20the%20polymer%20family%20under%20investigation%2C%20fluorination%20and%20functionalization%20by%20bulky%20alkyl%20side%20chains%20are%20both%20needed%20for%20reaching%20power%20conversion%20efficiencies%20above%2010%25.%22%2C%22date%22%3A%222018%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1039%5C%2Fc8ta04127j%22%2C%22ISSN%22%3A%222050-7488%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1039%5C%2Fc8ta04127j%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22TK3HH32E%22%2C%22BMA9GKQT%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222021-06-09T13%3A02%3A57Z%22%7D%7D%2C%7B%22key%22%3A%22WX36RUXN%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Howells%20et%20al.%22%2C%22parsedDate%22%3A%222016%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EC.T.%20Howells%2C%20K.%20Marbou%2C%20H.%20Kim%2C%20K.J.%20Lee%2C%20B.%20Heinrich%2C%20S.J.%20Kim%2C%20A.%20Nakao%2C%20T.%20Aoyama%2C%20S.%20Furukawa%2C%20J.-H.%20Kim%2C%20E.%20Kim%2C%20F.%20Mathevet%2C%20S.%20M%26%23xE9%3Bry%2C%20I.D.W.%20Samuel%2C%20A.%20Al%20Ghaferi%2C%20M.S.%20Dahlem%2C%20M.%20Uchiyama%2C%20S.Y.%20Kim%2C%20J.W.%20Wu%2C%20J.-C.%20Ribierre%2C%20C.%20Adachi%2C%20D.-W.%20Kim%2C%20P.%20Andre%2C%20Enhanced%20organic%20solar%20cells%20efficiency%20through%20electronic%20and%20electro-optic%20effects%20resulting%20from%20charge%20transfers%20in%20polymer%20hole%20transport%20blends%2C%20Journal%20of%20Materials%20Chemistry%20A%204%20%282016%29%204252%26%23x2013%3B4263.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fc6ta00677a%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fc6ta00677a%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Enhanced%20organic%20solar%20cells%20efficiency%20through%20electronic%20and%20electro-optic%20effects%20resulting%20from%20charge%20transfers%20in%20polymer%20hole%20transport%20blends%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Calvyn%20T.%22%2C%22lastName%22%3A%22Howells%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Khalid%22%2C%22lastName%22%3A%22Marbou%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Haeri%22%2C%22lastName%22%3A%22Kim%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Kwang%20Jin%22%2C%22lastName%22%3A%22Lee%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Benoit%22%2C%22lastName%22%3A%22Heinrich%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sang%20Jun%22%2C%22lastName%22%3A%22Kim%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Aiko%22%2C%22lastName%22%3A%22Nakao%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Tetsua%22%2C%22lastName%22%3A%22Aoyama%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Seiichi%22%2C%22lastName%22%3A%22Furukawa%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ju-Hyung%22%2C%22lastName%22%3A%22Kim%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Eunsun%22%2C%22lastName%22%3A%22Kim%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Fabrice%22%2C%22lastName%22%3A%22Mathevet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22M%5Cu00e9ry%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ifor%20D.%20W.%22%2C%22lastName%22%3A%22Samuel%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Amal%22%2C%22lastName%22%3A%22Al%20Ghaferi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Marcus%20S.%22%2C%22lastName%22%3A%22Dahlem%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Masanobu%22%2C%22lastName%22%3A%22Uchiyama%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sang%20Youl%22%2C%22lastName%22%3A%22Kim%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jeong%20Weon%22%2C%22lastName%22%3A%22Wu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jean-Charles%22%2C%22lastName%22%3A%22Ribierre%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Chihaya%22%2C%22lastName%22%3A%22Adachi%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Dong-Wook%22%2C%22lastName%22%3A%22Kim%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Pascal%22%2C%22lastName%22%3A%22Andre%22%7D%5D%2C%22abstractNote%22%3A%22We%20demonstrate%20that%20blending%20fluorinated%20molecules%20in%20PEDOT%3APSS%20hole%20transport%20layers%20%28HTL%29%20induces%20charge%20transfers%20which%20impact%20on%20both%20charge%20extraction%20and%20photogeneration%20within%20organic%20photovoltaic%20%28OPV%29%20devices.%20OPVs%20fabricated%20with%20modified%20HTL%20and%20two%20photoactive%20polymer%20blends%20led%20systematically%20to%20power%20conversion%20efficiencies%20%28PCE%29%20increases%2C%20with%20PTB7%3APC70BM%20blend%20exhibiting%20PCE%20of%20similar%20to%208.3%25%2C%20i.e.%20similar%20to%2015%25%20increase%20compared%20to%20pristine%20HTL%20devices.%20A%20reduced%20device-to-device%20characteristics%20variations%20was%20also%20noticed%20when%20fluorinated%20additives%20were%20used%20to%20modify%20the%20PEDOT%3A%20PSS.%20Shading%20lights%20onto%20the%20effect%20of%20HTL%20fluorination%2C%20we%20show%20that%20the%20morphology%20of%20the%20polymer%3A%20PCBM%20blends%20remains%20surprisingly%20unaffected%20by%20the%20fluorinated%20HTL%20surface%20energy%20but%20that%2C%20instead%2C%20the%20OPVs%20are%20impacted%20not%20only%20by%20the%20HTL%20electronic%20properties%20%28work%20function%2C%20dipole%20layer%2C%20open%20circuit%20voltage%2C%20charge%20transfer%20dynamic%29%20but%20also%20by%20alteration%20of%20the%20complex%20refractive%20indices%20%28photogeneration%2C%20short%20circuit%20current%20density%2C%20external%20quantum%20efficiencies%2C%20electro-optic%20modelling%29.%20Both%20mechanisms%20find%20their%20origin%20in%20fluorination%20induced%20charge%20transfers.%20This%20work%20points%20towards%20fluorination%20as%20a%20promising%20strategy%20toward%20combining%20both%20external%20quantum%20efficiency%20modulation%20and%20power%20conversion%20efficiency%20enhancement%20in%20OPVs.%20Charge%20transfers%20could%20also%20be%20used%20more%20broadly%20to%20tune%20the%20optical%20constants%20and%20electric%20field%20distribution%2C%20as%20well%20as%20to%20reduce%20interfacial%20charge%20recombinations%20within%20OPVs.%22%2C%22date%22%3A%222016%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1039%5C%2Fc6ta00677a%22%2C%22ISSN%22%3A%222050-7488%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1039%5C%2Fc6ta00677a%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22TK3HH32E%22%2C%22BMA9GKQT%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222021-06-09T13%3A01%3A49Z%22%7D%7D%2C%7B%22key%22%3A%22JQZD4FMN%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Han%20et%20al.%22%2C%22parsedDate%22%3A%222017%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ET.%20Han%2C%20I.%20Bulut%2C%20S.%20M%26%23xE9%3Bry%2C%20B.%20Heinrich%2C%20P.%20Leveque%2C%20N.%20Leclerc%2C%20T.%20Heiser%2C%20Improved%20structural%20order%20by%20side-chain%20engineering%20of%20organic%20small%20molecules%20for%20photovoltaic%20applications%2C%20Journal%20of%20Materials%20Chemistry%20C%205%20%282017%29%2010794%26%23x2013%3B10800.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fc7tc03155f%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fc7tc03155f%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Improved%20structural%20order%20by%20side-chain%20engineering%20of%20organic%20small%20molecules%20for%20photovoltaic%20applications%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22T.%22%2C%22lastName%22%3A%22Han%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22I.%22%2C%22lastName%22%3A%22Bulut%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22St%5Cu00e9phane%22%2C%22lastName%22%3A%22M%5Cu00e9ry%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Beno%5Cu00eet%22%2C%22lastName%22%3A%22Heinrich%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22P.%22%2C%22lastName%22%3A%22Leveque%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22N.%22%2C%22lastName%22%3A%22Leclerc%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22T.%22%2C%22lastName%22%3A%22Heiser%22%7D%5D%2C%22abstractNote%22%3A%22Despite%20obvious%20progress%20in%20organic%20semiconducting%20material%20design%20and%20organic%20bulk-heterojunction%20solar%20cell%20power%20conversion%20efficiencies%20the%20rationalization%20of%20the%20molecular%20design%20to%20finely%20tune%20organic%20semiconductor%20properties%20is%20still%20challenging.%20Herein%2C%20thanks%20to%20a%20particular%20dumbbell-shaped%20molecular%20design%20allowing%20partial%20decoupling%20between%20the%20structural%20properties%20and%20the%20frontier%20energy%20level%20positioning%20and%20optical%20absorption%20properties%2C%20we%20demonstrate%20the%20impact%20of%20the%20nature%20of%20side%20chains%20along%20the%20conjugated%20backbone%20on%20the%20structural%20properties%20of%20conjugated%20molecules.%20Thus%2C%20linear%20side%20chains%20on%20the%20structurally%20cohesive%20triazatruxene%20building%20blocks%20of%20our%20molecules%20provide%20higher%20stacking%20abilities%2C%20resulting%20in%20higher%20charge%20transport%20abilities%20and%20photovoltaic%20performances.%20These%20dumbbell-shaped%20molecules%20are%20a%20promising%20molecular%20family%20for%20reaching%20high%20solar%20cell%20efficiencies%20as%20well%20as%20for%20understanding%20in%20detail%20the%20impact%20of%20chemical%20structure%20on%20optoelectronic%20properties.%22%2C%22date%22%3A%222017%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1039%5C%2Fc7tc03155f%22%2C%22ISSN%22%3A%222050-7526%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1039%5C%2Fc7tc03155f%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22TK3HH32E%22%2C%22BMA9GKQT%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222021-06-09T13%3A02%3A42Z%22%7D%7D%2C%7B%22key%22%3A%22RDEU4IEG%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Guchait%20et%20al.%22%2C%22parsedDate%22%3A%222025%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3ES.%20Guchait%2C%20S.%20Oummouch%2C%20P.%20Durand%2C%20N.%20Kamatham%2C%20B.%20Jismy%2C%20L.%20Herrmann%2C%20S.%20M%26%23xE9%3Bry%2C%20N.%20Leclerc%2C%20M.%20Brinkmann%2C%20Impact%20of%20Side%20Chain%20Chemical%20Structure%20on%20Doping%20and%20Thermoelectric%20Properties%20of%20Oriented%20PBTTT%20Thin%20Films.%2C%20Small%2021%20%282025%29%202410073.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fsmll.202410073%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fsmll.202410073%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Impact%20of%20Side%20Chain%20Chemical%20Structure%20on%20Doping%20and%20Thermoelectric%20Properties%20of%20Oriented%20PBTTT%20Thin%20Films.%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Shubhradip%22%2C%22lastName%22%3A%22Guchait%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Said%22%2C%22lastName%22%3A%22Oummouch%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Pablo%22%2C%22lastName%22%3A%22Durand%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Narayanaswamy%22%2C%22lastName%22%3A%22Kamatham%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Badr%22%2C%22lastName%22%3A%22Jismy%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Laurent%22%2C%22lastName%22%3A%22Herrmann%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephane%22%2C%22lastName%22%3A%22M%5Cu00e9ry%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nicolas%22%2C%22lastName%22%3A%22Leclerc%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Martin%22%2C%22lastName%22%3A%22Brinkmann%22%7D%5D%2C%22abstractNote%22%3A%22In%20this%20contribution%2C%20doping%20of%20oriented%20thin%20films%20is%20investigated%20for%20three%20PBTTT%20polymers%20bearing%20different%20side%20chains%20including%20linear%20alkyl%20%5Cu2500%28CH2%2912%5Cu2500H%2C%20single%20ether%20%5Cu2500%28CH2%297%5Cu2500O%5Cu2500%28CH2%294%5Cu2500H%20and%20alkyl-siloxane%20%5Cu2500%28CH2%295%5Cu2500%28Si%28CH3%292O%292%5Cu2500Si%28CH3%293%20A%20combination%20of%20transmission%20electron%20microscopy%2C%20polarized%20UV-vis-NIR%20spectroscopy%20and%20transport%20measurements%20helps%20uncover%20the%20essential%20role%20of%20the%20chemical%20nature%20of%20side%20chains%20on%20the%20efficacy%20of%20the%20doping%20and%20on%20the%20resulting%20thermoelectric%20performances%20in%20oriented%20PBTTT%20films.%20Siloxane%20side%20chains%20help%20to%20reach%20record%20alignment%20level%20of%20PBTTT%20with%20dichroic%20ratio%20beyond%2050%20for%20an%20optimized%20rubbing%20temperature%20but%20they%20impede%20effective%20doping%20of%20PBTTT%20crystals%20with%20F6TCNNQ%2C%20resulting%20in%20very%20poor%20TE%20properties.%20By%20contrast%2C%20doping%20the%20amorphous%20phase%20of%20all%20three%20PBTTTs%20with%20magic%20blue%20%28MB%29%20results%20in%20excellent%20TE%20performances.%20Both%2C%20chemical%20nature%20of%20side%20chains%20and%20semi-crystalline%20structure%20of%20the%20polymer%20determine%20the%20efficacy%20of%20doping.%20The%20use%20of%20siloxane%20side%20chains%20further%20impacts%20the%20scaling%20laws%20Ssigma-1%5C%2Fs%20between%20the%20Seebeck%20coefficient%20S%20and%20the%20charge%20conductivity%20sigma.%20An%20unexpected%20s%20%3D%202%20exponent%20is%20observed%20and%20tentatively%20attributed%20to%20the%20dimensionality%20of%20charge%20transport%20in%20the%20highly%20oriented%20mesophase%20of%20PBTTT.%22%2C%22date%22%3A%222025%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1002%5C%2Fsmll.202410073%22%2C%22ISSN%22%3A%221613-6829%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222025-02-25T16%3A42%3A19Z%22%7D%7D%2C%7B%22key%22%3A%22TA8UVX5M%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Gr%5Cu00e9vin%20et%20al.%22%2C%22parsedDate%22%3A%222016%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EB.%20Gr%26%23xE9%3Bvin%2C%20P.-O.%20Schwartz%2C%20L.%20Biniek%2C%20M.%20Brinkmann%2C%20N.%20Leclerc%2C%20E.%20Zaborova%2C%20S.%20M%26%23xE9%3Bry%2C%20High-resolution%20noncontact%20AFM%20and%20Kelvin%20probe%20force%20microscopy%20investigations%20of%20self-assembled%20photovoltaic%20donor%26%23x2013%3Bacceptor%20dyads%2C%20Beilstein%20Journal%20of%20Nanotechnology%207%20%282016%29%20799%26%23x2013%3B808.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3762%5C%2Fbjnano.7.71%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.3762%5C%2Fbjnano.7.71%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22High-resolution%20noncontact%20AFM%20and%20Kelvin%20probe%20force%20microscopy%20investigations%20of%20self-assembled%20photovoltaic%20donor%5Cu2013acceptor%20dyads%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Benjamin%22%2C%22lastName%22%3A%22Gr%5Cu00e9vin%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Pierre-Olivier%22%2C%22lastName%22%3A%22Schwartz%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Laure%22%2C%22lastName%22%3A%22Biniek%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Martin%22%2C%22lastName%22%3A%22Brinkmann%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nicolas%22%2C%22lastName%22%3A%22Leclerc%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Elena%22%2C%22lastName%22%3A%22Zaborova%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22St%5Cu00e9phane%22%2C%22lastName%22%3A%22M%5Cu00e9ry%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222016%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.3762%5C%2Fbjnano.7.71%22%2C%22ISSN%22%3A%222190-4286%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.3762%5C%2Fbjnano.7.71%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22BMA9GKQT%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222021-06-09T13%3A01%3A35Z%22%7D%7D%2C%7B%22key%22%3A%22SWD5MN2S%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Fouchet%20et%20al.%22%2C%22parsedDate%22%3A%222018%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EJ.%20Fouchet%2C%20B.%20Heinrich%2C%20M.%20L%26%23x2019%3BHer%2C%20E.%20Voirin%2C%20L.%20Karmazin%2C%20C.%20Bailly%2C%20R.%20Welter%2C%20A.%20Mirjafari%2C%20L.%20Douce%2C%20Heterogeneous%20microwave-assisted%20Ullmann%20type%20methodology%20for%20synthesis%20of%20rigid-core%20ionic%20liquid%20crystals%2C%20New%20Journal%20of%20Chemistry%2042%20%282018%29%2010421%26%23x2013%3B10431.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fc8nj01609g%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1039%5C%2Fc8nj01609g%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Heterogeneous%20microwave-assisted%20Ullmann%20type%20methodology%20for%20synthesis%20of%20rigid-core%20ionic%20liquid%20crystals%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Julien%22%2C%22lastName%22%3A%22Fouchet%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Benoit%22%2C%22lastName%22%3A%22Heinrich%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matthieu%22%2C%22lastName%22%3A%22L%27Her%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Emilie%22%2C%22lastName%22%3A%22Voirin%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Lydia%22%2C%22lastName%22%3A%22Karmazin%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Corinne%22%2C%22lastName%22%3A%22Bailly%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Richard%22%2C%22lastName%22%3A%22Welter%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Arsalan%22%2C%22lastName%22%3A%22Mirjafari%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Laurent%22%2C%22lastName%22%3A%22Douce%22%7D%5D%2C%22abstractNote%22%3A%22We%20present%20an%20efficient%20Ullmann-type%20synthesis%20methodology%20enabling%20the%20preparation%20of%20imidazolium%20compounds%20with%20an%20extended%20aromatic%20core%20in%20three%20steps.%20This%20procedure%20begins%20with%20a%20microwave-assisted%2C%20heterogeneously%20catalysed%20cross-coupling%20reaction%20in%20the%20presence%20of%20Cu%28ii%29-doped%20NaY%20zeolite%20to%20obtain%20imidazole-aromatic%20derivatives%20directly%20in%20good%20yield.%20The%20innovation%20is%20that%20this%20reaction%20does%20not%20require%20solvent%2C%20ligand%20or%20an%20inert%20atmosphere.%20Subsequently%2C%20these%20derivatives%20have%20been%20alkylated%20with%20bromoalkanes%20and%20the%20bromide%20of%20the%20imidazolium%20products%20exchanged%20for%20%5BBF4%5D%28-%29%20or%20%5BPF6%5D%28-%29%20anions.%20We%20have%20studied%20the%20influence%20of%20the%20methyl%20group%20%28inductive%20effect%29%20on%20the%20aromatic%20unit%20during%20the%20coupling%20reaction%20as%20well%20as%20its%20impact%20on%20different%20arrangements%20in%20the%20crystalline%20state.%20We%20have%20been%20able%20to%20extend%20this%20synthesis%20to%20ionic%20liquid%20crystal%20compounds%20which%20display%20lamellar%20self-organization%20%28smectic%20A%29.%20The%20mesomorphic%20behavior%20and%20phase%20transition%20temperatures%20were%20investigated%20by%20polarizing%20optical%20microscopy%20%28POM%29%2C%20differential%20scanning%20calorimetry%20%28DSC%29%20and%20small-angle%20X-ray%20scattering%20%28SAXS%29.%22%2C%22date%22%3A%222018%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1039%5C%2Fc8nj01609g%22%2C%22ISSN%22%3A%221144-0546%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1039%5C%2Fc8nj01609g%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22TK3HH32E%22%2C%22BMA9GKQT%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222021-04-30T13%3A42%3A19Z%22%7D%7D%2C%7B%22key%22%3A%223G5MBFZK%22%2C%22library%22%3A%7B%22id%22%3A1839302%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Demangeat%20et%20al.%22%2C%22parsedDate%22%3A%222023%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%3Cdiv%20class%3D%5C%22csl-bib-body%5C%22%20style%3D%5C%22line-height%3A%201.35%3B%20%5C%22%3E%5Cn%20%20%3Cdiv%20class%3D%5C%22csl-entry%5C%22%20style%3D%5C%22clear%3A%20left%3B%20%5C%22%3E%5Cn%20%20%20%20%3Cdiv%20class%3D%5C%22csl-left-margin%5C%22%20style%3D%5C%22float%3A%20left%3B%20padding-right%3A%200.5em%3B%20text-align%3A%20right%3B%20width%3A%201em%3B%5C%22%3E%5B1%5D%3C%5C%2Fdiv%3E%3Cdiv%20class%3D%5C%22csl-right-inline%5C%22%20style%3D%5C%22margin%3A%200%20.4em%200%201.5em%3B%5C%22%3EC.%20Demangeat%2C%20Y.%20Tang%2C%20Y.%20Dou%2C%20S.%20Dale%2C%20J.%20Cielo%2C%20E.%20Kim%2C%20H.-J.%20Lee%2C%20A.%20D%26%23x2019%3BAleo%2C%20B.%20Hu%2C%20A.-J.%20Attias%2C%20Necessary%20and%20Sufficient%20Condition%20for%20Organic%20Room-Temperature%20Phosphorescence%20from%20Host-Guest%20Doped%20Crystalline%20Systems%2C%20Advanced%20Optical%20Materials%2011%20%282023%29%202300289.%20%3Ca%20class%3D%27zp-DOIURL%27%20href%3D%27https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fadom.202300289%27%3Ehttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1002%5C%2Fadom.202300289%3C%5C%2Fa%3E.%3C%5C%2Fdiv%3E%5Cn%20%20%3C%5C%2Fdiv%3E%5Cn%3C%5C%2Fdiv%3E%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Necessary%20and%20Sufficient%20Condition%20for%20Organic%20Room-Temperature%20Phosphorescence%20from%20Host-Guest%20Doped%20Crystalline%20Systems%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Catherine%22%2C%22lastName%22%3A%22Demangeat%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yipeng%22%2C%22lastName%22%3A%22Tang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yixuan%22%2C%22lastName%22%3A%22Dou%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sherrice%22%2C%22lastName%22%3A%22Dale%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jakob%22%2C%22lastName%22%3A%22Cielo%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Eunkyoung%22%2C%22lastName%22%3A%22Kim%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ha-Jin%22%2C%22lastName%22%3A%22Lee%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Anthony%22%2C%22lastName%22%3A%22D%27Aleo%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Bin%22%2C%22lastName%22%3A%22Hu%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Andre-Jean%22%2C%22lastName%22%3A%22Attias%22%7D%5D%2C%22abstractNote%22%3A%22Controlling%20and%20predicting%20the%20long-lived%20room-temperature%20phosphorescence%20%28RTP%29%20from%20organic%20materials%20are%20the%20next%20challenges%20to%20address%20for%20the%20realization%20of%20new%20efficient%20organic%20RTP%20systems.%20Here%2C%20a%20new%20approach%20is%20developed%20to%20reach%20these%20objectives%20by%20considering%20host-guest%20doped%20crystals%2C%20as%20well-suited%20model%20systems%20in%20that%20they%20allow%20the%20comprehensive%20understanding%20of%20synergetic%20structural%20interactions%20between%20crystalline%20host%20matrices%20and%20emitting%20guest%20molecules%2C%20one%20of%20the%20key%20parameters%20to%20understand%20the%20correlation%20between%20the%20solid-state%20organization%20and%20crystal%20RTP%20performances.%20Two%20series%20of%20s-conjugated%20donor%5C%2Facceptor%20%28D-s-A%29%20carbazole-based%20matrices%20and%20isomeric%201H-benzo%5Bf%5Dindole-based%20dopants%20are%20designed%2C%20capable%20of%20exploring%20a%20wide%20variety%20of%20conformations%20thanks%20to%20large%20rotational%20degrees%20of%20freedom%20provided%20by%20the%20s-conjugation.%20By%20correlating%20the%20results%20of%20single-crystal%20X-ray%20diffraction%20analysis%20and%20photoluminescence%20properties%2C%20a%20necessary%20and%20sufficient%20condition%20for%20RTP%20is%20established%20that%20paves%20the%20way%20for%20the%20development%20of%20new%20long-lived%20RTP%20host-guest%20doped%20systems.%22%2C%22date%22%3A%222023%22%2C%22language%22%3A%22English%22%2C%22DOI%22%3A%2210.1002%5C%2Fadom.202300289%22%2C%22ISSN%22%3A%222195-1071%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fdx.doi.org%5C%2F10.1002%5C%2Fadom.202300289%22%2C%22collections%22%3A%5B%222DH6J37C%22%2C%22VYTETDZF%22%5D%2C%22dateModified%22%3A%222023-06-15T14%3A47%3A12Z%22%7D%7D%5D%7D
[1]
X. Zhu, C. Hessin, A. Salame, L. Sosa-Vargas, D. Kreher, C. Adachi, A. Proust, P. Mialane, J. Marrot, A. Bouchet, M. Sliwa, S. Méry, B. Heinrich, F. Mathevet, G. Izzet, Photoactive Organic/Inorganic Hybrid Materials with Nanosegregated Donor-Acceptor Arrays, Angewandte Chemie-International Edition 60 (2021) 8419–8424. https://doi.org/10.1002/anie.202014319.
[1]
E. Zaborova, P. Chavez, R. Bechara, P. Leveque, T. Heiser, S. Méry, N. Leclerc, Thiazole as a weak electron-donor unit to lower the frontier orbital energy levels of donor-acceptor alternating conjugated materials, Chemical Communications 49 (2013) 9938–9940. https://doi.org/10.1039/c3cc45481a.
[1]
W. Yu, C.A. Serra, I.U. Khan, M. Er-Rafik, M. Schmutz, I. Kraus, S. Ding, L. Zhang, M. Bouquey, R. Muller, Development of an Elongational-Flow Microprocess for the Production of Size-Controlled Nanoemulsions: Application to the Preparation of Monodispersed Polymer Nanoparticles and Composite Polymeric Microparticles, Macromolecular Reaction Engineering 11 (2017) 1600025. https://doi.org/10.1002/mren.201600025.
[1]
W. Yu, N. Visaveliya, C.A. Serra, J.M. Köhler, S. Ding, M. Bouquey, R. Muller, M. Schmutz, I. Kraus, Preparation and Deep Characterization of Composite/Hybrid Multi-Scale and Multi-Domain Polymeric Microparticles, Materials 12 (2019) 3921. https://doi.org/10.3390/ma12233921.
[1]
J. Shaya, J.-C. Ribierre, G. Correia, Y.J. Dappe, F. Mathevet, L. Mager, B. Heinrich, S. Méry, Control of the Organization of 4,4’-bis(carbazole)-1,1’-biphenyl (CBP) Molecular Materials through Siloxane Functionalization., Molecules 28 (2023) 2038. https://doi.org/10.3390/molecules28052038.
[1]
J. Shaya, G. Correia, B. Heinrich, J.-C. Ribierre, K. Polychronopoulou, L. Mager, S. Méry, Functionalization of Biphenylcarbazole (CBP) with Siloxane-Hybrid Chains for Solvent-Free Liquid Materials, Molecules 27 (2022) 89. https://doi.org/10.3390/molecules27010089.
[1]
C.A. Serra, I.U. Khan, Z.Q. Chang, M. Bouquey, R. Muller, I. Kraus, M. Schmutz, T. Vandamme, N. Anton, C. Ohm, R. Zentel, A. Knauer, M. Kohler, Engineering Polymer Microparticles by Droplet Microfluidics, Journal of Flow Chemistry 3 (2013) 66–75. https://doi.org/10.1556/jfc-d-13-00014.
[1]
P.-O. Schwartz, F. Moingeon, J. Roeser, E. Couzigne, E. Voirin, P. Masson, S. Méry, Preparation of multi-allylic dendronized polymers via atom-transfer radical polymerization, European Polymer Journal 118 (2019) 358–364. https://doi.org/10.1016/j.eurpolymj.2019.06.009.
[1]
P.O. Schwartz, E. Zaborova, R. Bechara, P. Leveque, T. Heiser, S. Méry, N. Leclerc, Impact of the arrangement of functional moieties within small molecular systems for solution processable bulk heterojunction solar cells, New Journal of Chemistry 37 (2013) 2317–2323. https://doi.org/10.1039/c3nj00218g.
[1]
P.-O. Schwartz, L. Biniek, E. Zaborova, B. Heinrich, M. Brinkrnann, N. Leclerc, S. Méry, Perylenediimide-Based Donor-Acceptor Dyads and Triads: Impact of Molecular Architecture on Self-Assembling Properties, Journal of the American Chemical Society 136 (2014) 5981–5992. https://doi.org/10.1021/ja4129108.
[1]
T. Roland, G.H. Ramirez, J. Léonard, S. Méry, S. Haacke, Ultrafast broadband laser spectroscopy reveals energy and charge transfer in novel donor-acceptor triads for photovoltaic applications, in: Ye, C and Wang, ZL and Zhou, B (Ed.), Journal of Physics: Conference Series, 2011: p. 012006 /p. 1–6. https://doi.org/10.1088/1742-6596/276/1/012006.
[1]
T. Roland, J. Léonard, G.H. Ramirez, S. Méry, O. Yurchenko, S. Ludwigs, S. Haacke, Sub-100 fs charge transfer in a novel donor-acceptor-donor triad organized in a smectic film, Physical Chemistry Chemical Physics 14 (2012) 273–279. https://doi.org/10.1039/c1cp22122a.
[1]
J. Roeser, F. Moingeon, B. Heinrich, P. Masson, F. Arnaud-Neu, M. Rawiso, S. Méry, Dendronized Polymers with Peripheral Oligo(ethylene oxide) Chains: Thermoresponsive Behavior and Shape Anisotropy in Solution, Macromolecules 44 (2011) 8925–8935. https://doi.org/10.1021/ma2016776.
[1]
J. Roeser, B. Heinrich, C. Bourgogne, M. Rawiso, S. Michel, V. Hubscher-Bruder, F. Arnaud-Neu, S. Méry, Dendronized Polymers with Silver and Mercury Cations Recognition: Complexation Studies and Polyelectrolyte Behavior, Macromolecules (Washington, DC, U. S.) 46 (2013) 7075–7085. https://doi.org/10.1021/ma400348v.
[1]
J.-C. Ribierre, Z. Li, X. Liu, E. Lacaze, B. Heinrich, S. Méry, P. Sleczkowski, Y. Xiao, F. Lafolet, D. Hashizume, T. Aoyama, M. Uchiyama, J.W. Wu, E. Zaborova, F. Fages, A. D’Aleo, F. Mathevet, C. Adachi, A solvent-free and vacuum-free melt-processing method to fabricate organic semiconducting layers with large crystal size for organic electronic applications, J. Mater. Chem. C (2019). https://doi.org/10.1039/C8TC04834G.
[1]
J.-C. Ribierre, T. Tanaka, L. Zhao, Y. Yokota, S. Matsumoto, D. Hashizume, K. Takaishi, T. Muto, B. Heinrich, S. Méry, F. Mathevet, T. Matsushima, M. Uchiyama, C. Adachi, T. Aoyama, Simultaneous Edge-on to Face-on Reorientation and 1D Alignment of Small pi-Conjugated Molecules Using Room-Temperature Mechanical Rubbing, Advanced Functional Materials 28 (2018). https://doi.org/10.1002/adfm.201707038.
[1]
J.-C. Ribierre, L. Zhao, M. Inoue, P.-O. Schwartz, J.-H. Kim, K. Yoshida, A.S.D. Sandanayaka, H. Nakanotani, L. Mager, S. Méry, C. Adachi, Low threshold amplified spontaneous emission and ambipolar charge transport in non-volatile liquid fluorene derivatives, Chemical Communications 52 (2016) 3103–3106. https://doi.org/10.1039/c5cc08331a.
[1]
M. Polkehn, P. Eisenbrandt, H. Tamura, S. Haacke, S. Méry, I. Burghardt, Ultrafast excitonic and charge transfer dynamics in nanostructured organic polymer materials, in: Andrews, DL and Nunzi, JM and Ostendorf, A (Ed.), NANOPHOTONICS VI, SPIE-INT SOC OPTICAL ENGINEERING, 1000 20TH ST, PO BOX 10, BELLINGHAM, WA 98227-0010 USA, 2016. https://doi.org/10.1117/12.2230314.
[1]
M. Polkehn, H. Tamura, P. Eisenbrandt, S. Haacke, S. Méry, I. Burghardt, Molecular Packing Determines Charge Separation in a Liquid Crystalline Bisthiophene–Perylene Diimide Donor–Acceptor Material, Journal of Physical Chemistry Letters 7 (2016) 1327–1334. https://doi.org/10.1021/acs.jpclett.6b00277.
[1]
T. Olla, R. Jabbour, A. Labiod, O. Boyron, S. Méry, B. Heinrich, T. Heiser, D. Jacquemin, P. Leveque, A. Lesage, N. Leclerc, How Halogenation Impacts the Polymer Backbone Conformation: Learning from Combination of Solid-State MAS NMR and X-Ray Scattering, Advanced Functional Materials 32 (2022) 2204929. https://doi.org/10.1002/adfm.202204929.
[1]
T. Olla, O.A. Ibraikulov, S. Ferry, O. Boyron, S. Méry, B. Heinrich, T. Heiser, P. Lévêque, N. Leclerc, Benzothiadiazole Halogenation Impact in Conjugated Polymers, a Comprehensive Study, Macromolecules 52 (2019) 8006–8016. https://doi.org/10.1021/acs.macromol.9b01760.
[1]
S.T. Nestor, B. Heinrich, R.A. Sykora, X. Zhang, G.J. McManus, L. Douce, A. Mirjafari, Methimazolium-based ionic liquid crystals: Emergence of mesomorphic properties via a sulfur motif, Tetrahedron 73 (2017) 5456–5460. https://doi.org/https://doi.org/10.1016/j.tet.2017.07.056.
[1]
F. Mouillard, T. Ferté, E. Voirin, S. Méry, P. Masson, A. Carradò, Use of a Photocleavable Initiator to Characterize Polymer Chains Grafted onto a Metal Plate with the Grafting-from Method., Polymers 15 (2023) 1265. https://doi.org/10.3390/polym15051265.
[1]
S. Marzuk, B. Heinrich, P. Leveque, N. Leclerc, J. Khiari, S. Méry, Phthalocyanine-based dumbbell-shaped molecule: Synthesis, structure and charge transport studies, Dyes and Pigments 154 (2018) 282–289. https://doi.org/10.1016/j.dyepig.2018.03.017.
[1]
S. Marzouk, A. Khalfallah, B. Heinrich, J.E. Khiari, A. Kriaa, S. Méry, Synthesis and mesomorphic properties of liquid crystals containing a perfluorinated segment via different linkers, Journal of Fluorine Chemistry 197 (2017) 15–23. https://doi.org/10.1016/j.jfluchem.2017.02.006.
[1]
C. Mahmoudi, I. Bulut, J. Jing, S. Fall, B. Heinrich, S. Méry, T. Heiser, P. Leveque, E. Steveler, M. Majdoub, N. Leclerc, Regioisomers of Organic Semiconducting Dumbbell-Shaped Molecules: Synthesis and Structure-Properties Relationship, European Journal of Organic Chemistry (2021) 3170–3177. https://doi.org/10.1002/ejoc.202100473.
[1]
X. Liu, V. Placide, L. Chu, K.M. Haidaraly, L.S. Vargas, C. Adachi, J.W. Wu, B. Heinrich, E. Lacaze, W. Yan, A. D’Aleo, F. Mathevet, Investigation and modulation of charge transport properties with thin films of an isoindigo-based donor-acceptor molecular semiconductor, Applied Surface Science 686 (2025) 162057. https://doi.org/10.1016/j.apsusc.2024.162057.
[1]
L. Liu, P. Eisenbrandt, T. Roland, M. Polkehn, P.-O. Schwartz, K. Bruchlos, B. Omiecienski, S. Ludwigs, N. Leclerc, E. Zaborova, J. Léonard, S. Méry, I. Burghardt, S. Haacke, Controlling Charge Separation and Recombination by Chemical Design in Donor-Acceptor Dyads, Physical Chemistry Chemical Physics 18 (2016) 18536–18548. https://doi.org/10.1039/C6CP00644B.
[1]
K.J. Lee, J.H. Woo, Y. Xiao, E. Kim, L. Mazur, D. Kreher, A.-J. Attias, K. Matczyszyn, M. Samoc, B. Heinrich, S. Méry, F. Fages, L. Mager, A. D’Aleo, J.W. Wu, F. MATHEVET, P. Andre, J.-C. Ribierre, Structure-charge transfer property relationship in self-assembled discotic liquid-crystalline donor-acceptor dyad and triad thin films, RSC Advances 6 (2016) 57811–57819. https://doi.org/10.1039/C6RA08039A.
[1]
A. Labiod, O.A. Ibraikulov, S. Dabos-Seignon, S. Ferry, B. Heinrich, S. Méry, S. Fall, H.J.T. Nkuissi, T. Heiser, C. Cabanetos, N. Leclerc, P. Leveque, Photo-degradation in bulk heterojunction organic solar cells using a fullerene or a non-fullerene derivative electron acceptor, Organic Electronics 107 (2022) 106549. https://doi.org/10.1016/j.orgel.2022.106549.
[1]
M. L’Her, Y. Atoini, J. Fouchet, B. Heinrich, N. Del-Giudice, E. Scrafton, E. Bordes, L. Karmazin, L. Charbonniere, L. De Cola, L. Douce, Luminescent imidazolium-naphthalene salts in liquid and solid states (vol 43, pg 12529, 2019), New Journal of Chemistry 44 (2020) 2669. https://doi.org/10.1039/d0nj90011g.
[1]
M. L’Her, Y. Atoini, J. Fouchet, B. Heinrich, N. Del-Giudice, E. Scrafton, E. Bordes, L. Karmazin, L. Charboniere, L. De Cola, L. Douce, Luminescent imidazolium-naphthalene salts in liquid and solid states, New Journal of Chemistry 43 (2019) 12529–12532. https://doi.org/10.1039/c9nj02972a.
[1]
K.S. Kumar, N. Del Giudice, B. Heinrich, L. Douce, M. Ruben, Bistable spin-crossover in a new series of [Fe(BPP-R)(2)](2+) (BPP=2,6-bis(pyrazol-1-yl)pyridine; R = CN) complexes, Dalton Transactions 49 (2020) 14258–14267. https://doi.org/10.1039/d0dt02214d.
[1]
I. Kraus, S. Li, A. Knauer, M. Schmutz, J. Faerber, C.A. Serra, M. Kohler, Continuous-Microflow Synthesis and Morphological Characterization of Multiscale Composite Materials Based on Polymer Microparticles and Inorganic Nanoparticles, Journal of Flow Chemistry 4 (2014) 72–78. https://doi.org/10.1556/JFC-D-13-00029.
[1]
J.M. Köhler, A. März, J. Popp, A. Knauer, I. Kraus, J. Faerber, C. Serra, Polyacrylamid/Silver Composite Particles Produced via Microfluidic Photopolymerization for Single Particle-Based SERS Microsensorics, Analytical Chemistry 85 (2013) 313–318. https://doi.org/10.1021/ac302751t.
[1]
J.M. Köhler, I. Kraus, J. Faerber, C. Serra, Continuous-flow preparation of nanoporous metal/polymer composite particles by in situ synthesis of silver nanoparticles in photopolymerized acrylate/diethylene glycol droplets, Journal of Materials Science 48 (2013) 2158–2166. https://doi.org/10.1007/s10853-012-6991-0.
[1]
M. Kim, K.R. Choi, Y.U. Lee, B. Heinrich, S.Y. Ko, F. Mathevet, J.-C. Ribierre, A. D’Aleo, J.W. Wu, V. Placide, Natural Hyperbolic Dispersion with Anisotropic Epsilon-Near-Zero and Epsilon-Near-Pole in Squaraine Molecular Film, Advanced Optical Materials (2021) 2101091. https://doi.org/10.1002/adom.202101091.
[1]
I.U. Khan, C.A. Serra, N. Anton, M. Er-Rafik, C. Blanck, M. Schmutz, I. Kraus, N. Messaddeq, C. Sutter, H. Anton, A.S. Klymchenko, T.F. Vandamme, Microfluidic conceived Trojan microcarriers for oral delivery of nanoparticles, International Journal of Pharmaceutics 493 (2015) 7–15. https://doi.org/10.1016/j.ijpharm.2015.06.028.
[1]
I.U. Khan, C.A. Serra, N. Anton, X. Li, R. Akasov, N. Messaddeq, I. Kraus, T.F. Vandamme, Microfluidic conceived drug loaded Janus particles in side-by-side capillaries device, International Journal of Pharmaceutics 473 (2014) 239–249. https://doi.org/10.1016/j.ijpharm.2014.06.035.
[1]
N. Kamatham, O.A. Ibraikulov, P. Durand, J. Wang, O. Boyron, B. Heinrich, T. Heiser, P. Leveque, N. Leclerc, S. Méry, On the Impact of Linear Siloxanated Side Chains on the Molecular Self-Assembling and Charge Transport Properties of Conjugated Polymers, Advanced Functional Materials 31 (2021) 2007734. https://doi.org/10.1002/adfm.202007734.
[1]
J. Jing, E. Steveler, N. Leclerc, A. D’Aleo, B. Heinrich, W. Uhring, T. Heiser, Exciton lifetime in donor-acceptor-donor planar dumbbell-shaped triazatruxene-thienopyrroledione derivatives, in: S. Reineke, K. Vandewal, W. Maes (Eds.), ORGANIC ELECTRONICS AND PHOTONICS: FUNDAMENTALS AND DEVICES III, SPIE-INT SOC OPTICAL ENGINEERING, 2022. https://doi.org/10.1117/12.2615963.
[1]
J. Jing, B. Heinrich, A. Prel, E. Steveler, T. Han, I. Bulut, S. Méry, Y. Leroy, N. Leclerc, P. Leveque, M. Rosenthal, D.A. Ivanov, T. Heiser, Efficient 3D charge transport in planar triazatruxene-based dumbbell-shaped molecules forming a bridged columnar phase, Journal of Materials Chemistry A 9 (2021) 24315–24324. https://doi.org/10.1039/d1ta06300f.
[1]
Olzhas.A. Ibraikulov, J. Wang, N. Kamatham, B. Heinrich, S. Méry, M. Kohlstädt, U. Würfel, S. Ferry, N. Leclerc, T. Heiser, P. Lévêque, ITO-Free Organic Photovoltaic Modules Based on Fluorinated Polymers Deposited from Non-Halogenated Solution: A Major Step Toward Large-Scale Module Production, Solar RRL 3 (2019) 1900273. https://doi.org/10.1002/solr.201900273.
[1]
O.A. Ibraikulov, C. Ngov, P. Chavez, I. Bulut, B. Heinrich, O. Boyron, K.L. Gerasimov, D.A. Ivanov, S. Swaraj, S. Méry, N. Leclerc, P. Leveque, T. Heiser, Face-on orientation of fluorinated polymers conveyed by long alkyl chains: a prerequisite for high photovoltaic performances, Journal of Materials Chemistry A 6 (2018) 12038–12045. https://doi.org/10.1039/c8ta04127j.
[1]
C.T. Howells, K. Marbou, H. Kim, K.J. Lee, B. Heinrich, S.J. Kim, A. Nakao, T. Aoyama, S. Furukawa, J.-H. Kim, E. Kim, F. Mathevet, S. Méry, I.D.W. Samuel, A. Al Ghaferi, M.S. Dahlem, M. Uchiyama, S.Y. Kim, J.W. Wu, J.-C. Ribierre, C. Adachi, D.-W. Kim, P. Andre, Enhanced organic solar cells efficiency through electronic and electro-optic effects resulting from charge transfers in polymer hole transport blends, Journal of Materials Chemistry A 4 (2016) 4252–4263. https://doi.org/10.1039/c6ta00677a.
[1]
T. Han, I. Bulut, S. Méry, B. Heinrich, P. Leveque, N. Leclerc, T. Heiser, Improved structural order by side-chain engineering of organic small molecules for photovoltaic applications, Journal of Materials Chemistry C 5 (2017) 10794–10800. https://doi.org/10.1039/c7tc03155f.
[1]
S. Guchait, S. Oummouch, P. Durand, N. Kamatham, B. Jismy, L. Herrmann, S. Méry, N. Leclerc, M. Brinkmann, Impact of Side Chain Chemical Structure on Doping and Thermoelectric Properties of Oriented PBTTT Thin Films., Small 21 (2025) 2410073. https://doi.org/10.1002/smll.202410073.
[1]
B. Grévin, P.-O. Schwartz, L. Biniek, M. Brinkmann, N. Leclerc, E. Zaborova, S. Méry, High-resolution noncontact AFM and Kelvin probe force microscopy investigations of self-assembled photovoltaic donor–acceptor dyads, Beilstein Journal of Nanotechnology 7 (2016) 799–808. https://doi.org/10.3762/bjnano.7.71.
[1]
J. Fouchet, B. Heinrich, M. L’Her, E. Voirin, L. Karmazin, C. Bailly, R. Welter, A. Mirjafari, L. Douce, Heterogeneous microwave-assisted Ullmann type methodology for synthesis of rigid-core ionic liquid crystals, New Journal of Chemistry 42 (2018) 10421–10431. https://doi.org/10.1039/c8nj01609g.
[1]
C. Demangeat, Y. Tang, Y. Dou, S. Dale, J. Cielo, E. Kim, H.-J. Lee, A. D’Aleo, B. Hu, A.-J. Attias, Necessary and Sufficient Condition for Organic Room-Temperature Phosphorescence from Host-Guest Doped Crystalline Systems, Advanced Optical Materials 11 (2023) 2300289. https://doi.org/10.1002/adom.202300289.
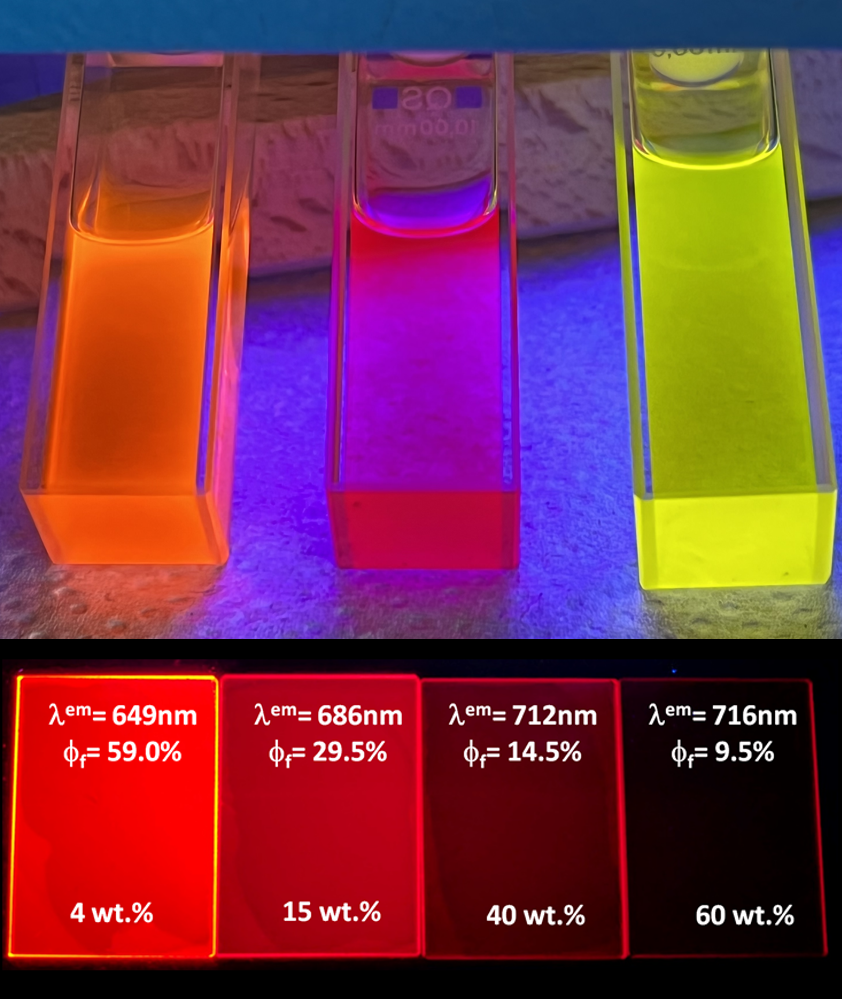
Photophysique et photonique pour l'électronique organique